Abacavir Increases Purinergic P2X7 Receptor Activation by ATP: Does a Pro-inflammatory Synergism Underlie Its Cardiovascular Toxicity?
- PMID: 33867979
- PMCID: PMC8045785
- DOI: 10.3389/fphar.2021.613449
Abacavir Increases Purinergic P2X7 Receptor Activation by ATP: Does a Pro-inflammatory Synergism Underlie Its Cardiovascular Toxicity?
Abstract
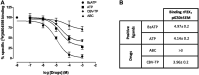
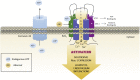
The cardiovascular toxicity of Abacavir is related to its purinergic structure. Purinergic P2X7-receptors (P2X7R), characterized by activation by high concentrations of ATP and with high plasticity, seem implicated. We appraise the nature of the interplay between Abacavir and P2X7R in generating vascular inflammation. The effects of Abacavir on leukocyte-endothelium interactions were compared with those of its metabolite carbovir triphosphate (CBV-TP) or ATP in the presence of apyrase (ATP-ase) or A804598 (P2X7R-antagonist). CBV-TP and ATP levels were evaluated by HPLC, while binding of Abacavir, CBV-TP and ATP to P2X7R was assessed by radioligand and docking studies. Hypersensitivity studies explored a potential allosteric action of Abacavir. Clinical concentrations of Abacavir (20 µmol/L) induced leukocyte-endothelial cell interactions by specifically activating P2X7R, but the drug did not show affinity for the P2X7R ATP-binding site (site 1). CBV-TP levels were undetectable in Abacavir-treated cells, while those of ATP were unaltered. The effects of Abacavir were Apyrase-dependent, implying dependence on endogenous ATP. Exogenous ATP induced a profile of proinflammatory actions similar to Abacavir, but was not entirely P2X7R-dependent. Docking calculations suggested ATP-binding to sites 1 and 2, and Abacavir-binding only to allosteric site 2. A combination of concentrations of Abacavir (1 µmol/L) and ATP (0.1 µmol/L) that had no effect when administered separately induced leukocyte-endothelium interactions mediated by P2X7R and involving Connexin43 channels. Therefore, Abacavir acts as a positive allosteric modulator of P2X7R, turning low concentrations of endogenous ATP themselves incapable of stimulating P2X7R into a functional proinflammatory agonist of the receptor.
Keywords: P2X7 receptor; abacavir; adenosine triphosphate; allosteric modulator; cardiovascular diseases; leukocyte-endothelium interactions.
Copyright © 2021 Collado-Díaz, Martinez-Cuesta, Blanch-Ruiz, Sánchez-López, García-Martínez, Peris, Usach, Ivorra, Lacetera, Martín-Santamaría, Esplugues and Alvarez.
Conflict of interest statement
JVE has received funds for speaking at symposia organized by Abbvie Farmaceutica, Astra Zeneca, Gilead Sciences and Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Alvarez A., Rios-Navarro C., Blanch-Ruiz M. A., Collado-Diaz V., Andujar I., Martinez-Cuesta M. A., et al. (2017). Abacavir induces platelet-endothelium interactions by interfering with purinergic signalling: a step from inflammation to thrombosis. Antiviral Res. 141, 179–185. 10.1016/j.antiviral.2017.03.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

