An Overview of Antennal Esterases in Lepidoptera
- PMID: 33868009
- PMCID: PMC8044547
- DOI: 10.3389/fphys.2021.643281
An Overview of Antennal Esterases in Lepidoptera
Abstract
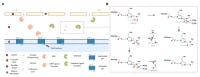
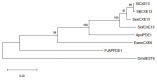
Lepidoptera are used as a model for the study of insect olfactory proteins. Among them, odorant degrading enzymes (ODEs), that degrade odorant molecules to maintain the sensitivity of antennae, have received less attention. In particular, antennal esterases (AEs; responsible for ester degradation) are crucial for intraspecific communication in Lepidoptera. Currently, transcriptomic and genomic studies have provided AEs in several species. However, efforts in gene annotation, classification, and functional assignment are still lacking. Therefore, we propose to combine evidence at evolutionary, structural, and functional level to update ODEs as well as key information into an easier classification, particularly of AEs. Finally, the kinetic parameters for putative inhibition of ODEs are discussed in terms of its role in future integrated pest management (IPM) strategies.
Keywords: Lepidoptera; antennal esterases; inhibition; olfactory system; semiochemicals; transcriptomic.
Copyright © 2021 Godoy, Machuca, Venthur, Quiroz and Mutis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All the information performed in this work is of a public nature. We created the figures and the data generated in relation to the transcripts identified from the antennal transcriptome of E. semipurpurella.
Figures

Similar articles
-
Identification of candidate odorant degrading gene/enzyme systems in the antennal transcriptome of Drosophila melanogaster.Insect Biochem Mol Biol. 2014 Oct;53:30-43. doi: 10.1016/j.ibmb.2014.07.003. Epub 2014 Jul 16. Insect Biochem Mol Biol. 2014. PMID: 25038463
-
Characterization of Two Aldehyde Oxidases from the Greater Wax Moth, Galleria mellonella Linnaeus. (Lepidoptera: Pyralidae) with Potential Role as Odorant-Degrading Enzymes.Insects. 2022 Dec 12;13(12):1143. doi: 10.3390/insects13121143. Insects. 2022. PMID: 36555053 Free PMC article.
-
Antennal transcriptome analyses and olfactory protein identification in an important wood-boring moth pest, Streltzoviella insularis (Lepidoptera: Cossidae).Sci Rep. 2019 Nov 29;9(1):17951. doi: 10.1038/s41598-019-54455-w. Sci Rep. 2019. PMID: 31784624 Free PMC article.
-
Sex pheromones and olfactory proteins in Antheraea moths: A. pernyi and A. polyphemus (Lepidoptera: Saturniidae).Arch Insect Biochem Physiol. 2020 Oct;105(2):e21729. doi: 10.1002/arch.21729. Epub 2020 Aug 6. Arch Insect Biochem Physiol. 2020. PMID: 32761939 Review.
-
Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes.Annu Rev Entomol. 2013;58:373-91. doi: 10.1146/annurev-ento-120811-153635. Epub 2012 Sep 27. Annu Rev Entomol. 2013. PMID: 23020622 Review.
Cited by
-
Genetic mechanisms modulating behaviour through plastic chemosensory responses in insects.Mol Ecol. 2023 Jan;32(1):45-60. doi: 10.1111/mec.16739. Epub 2022 Nov 2. Mol Ecol. 2023. PMID: 36239485 Free PMC article. Review.
-
Identification of metabolizing enzyme genes associated with xenobiotics and odorants in the predatory stink bug Arma custos based on transcriptome analysis.Heliyon. 2023 Jul 27;9(8):e18657. doi: 10.1016/j.heliyon.2023.e18657. eCollection 2023 Aug. Heliyon. 2023. PMID: 37576196 Free PMC article.
-
Transcriptomic Identification and Expression Profile Analysis of Odorant-Degrading Enzymes from the Asian Corn Borer Moth, Ostrinia furnacalis.Insects. 2022 Nov 6;13(11):1027. doi: 10.3390/insects13111027. Insects. 2022. PMID: 36354851 Free PMC article.
-
Comparative expression profiles of carboxylesterase orthologous CXE14 in two closely related tea geometrid species, Ectropis obliqua Prout and Ectropis grisescens Warren.Front Physiol. 2023 May 24;14:1194997. doi: 10.3389/fphys.2023.1194997. eCollection 2023. Front Physiol. 2023. PMID: 37293262 Free PMC article.
-
Antennal transcriptomic analysis of carboxylesterases and glutathione S-transferases associated with odorant degradation in the tea gray geometrid, Ectropis grisescens (Lepidoptera, Geometridae).Front Physiol. 2023 Apr 4;14:1183610. doi: 10.3389/fphys.2023.1183610. eCollection 2023. Front Physiol. 2023. PMID: 37082242 Free PMC article.
References
-
- Ando T., Inomata S. I., Yamamoto M. (2004). “Lepidopteran sex pheromones” in The chemistry of pheromones and other semiochemicals I. ed. Schulz S. (Berlin, Heidelberg: Springer; ), 51–96. - PubMed
-
- Arn H., Tóth M., Priesner E. (1992). List of sex pheromones of Lepidoptera and related attractants. 2nd Edn. Montfavet, France: International Organization for Biological Control.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

