ATP1A3-Related Disorders: An Ever-Expanding Clinical Spectrum
- PMID: 33868146
- PMCID: PMC8047318
- DOI: 10.3389/fneur.2021.637890
ATP1A3-Related Disorders: An Ever-Expanding Clinical Spectrum
Abstract
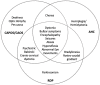
The Na+/K+ ATPases are Sodium-Potassium exchanging pumps, with a heteromeric α-β-γ protein complex. The α3 isoform is required as a rescue pump, after repeated action potentials, with a distribution predominantly in neurons of the central nervous system. This isoform is encoded by the ATP1A3 gene. Pathogenic variants in this gene have been implicated in several phenotypes in the last decades. Carriers of pathogenic variants in this gene manifest neurological and non-neurological features in many combinations, usually with an acute onset and paroxysmal episodes triggered by fever or other factors. The first three syndromes described were: (1) rapid-onset dystonia parkinsonism; (2) alternating hemiplegia of childhood; and, (3) cerebellar ataxia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS syndrome). Since their original description, an expanding number of cases presenting with atypical and overlapping features have been reported. Because of this, ATP1A3-disorders are now beginning to be viewed as a phenotypic continuum representing discrete expressions along a broadly heterogeneous clinical spectrum.
Keywords: ATP1A3; CAPOS syndrome; Dyt12; alternating hemiplegia; ataxia; rapid-onset dystonia parkinsonism; sodium-potassium-exchanging ATPase.
Copyright © 2021 Salles, Mata, Brünger, Lal and Fernandez.
Conflict of interest statement
IM and HF Grants/Research Support. IM has received research support from American Parkinson's Disease Association, Parkinson's Foundation, Michael J. Fox Foundation and NIH/NINDS. IF has received research support from Acorda Therapeutics, Alkahest, Amneal, Biogen, Michael J. Fox Foundation, Movement Disorders Society, NIH/NINDS, Parkinson Study Group, Sunovion, but has no owner interest in any pharmaceutical company. HF has received honoraria from, Cleveland Clinic, Boston University, as a speaker in CME events. HF has received honoraria from Bial Neurology, Biopas, Cerevel, CNS Ratings, Denali Therapeutics, Kyowa Hakko Kirin, Pfizer, Partners Healthcare System, Parkinson Study Group, Revance, Sun Pharmaceutical Industries, Sunovion Research and Development Trust as a consultant. Elsevier as the Co-Editor-In-Chief of Parkinsonism and Related Disorders Journal. Royalty: HF has received royalty payments from Demos Publishing and Springer for serving as a book author/editor. Contractual Services: The Cleveland Clinic has a contract with Teva for HF role as a Co-Principal Investigator in Deutetrabenazine for Tardive Dyskinesia global studies. DL receives funds from NIH NINDS, Friends of FACES, German Research Foundation, Federal Ministry of Education and Research (BMBF). The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

