Short-Term High-Fat Feeding Does Not Alter Mitochondrial Lipid Respiratory Capacity but Triggers Mitophagy Response in Skeletal Muscle of Mice
- PMID: 33868178
- PMCID: PMC8044530
- DOI: 10.3389/fendo.2021.651211
Short-Term High-Fat Feeding Does Not Alter Mitochondrial Lipid Respiratory Capacity but Triggers Mitophagy Response in Skeletal Muscle of Mice
Abstract
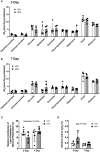
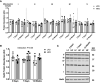
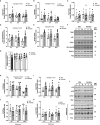
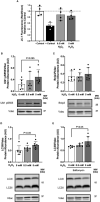
Lipid overload of the mitochondria is linked to the development of insulin resistance in skeletal muscle which may be a contributing factor to the progression of type 2 diabetes during obesity. The targeted degradation of mitochondria through autophagy, termed mitophagy, contributes to the mitochondrial adaptive response to changes in dietary fat. Our previous work demonstrates long-term (2-4 months) consumption of a high-fat diet increases mitochondrial lipid oxidation capacity but does not alter markers of mitophagy in mice. The purpose of this study was to investigate initial stages of mitochondrial respiratory adaptations to high-fat diet and the activation of mitophagy. C57BL/6J mice consumed either a low-fat diet (LFD, 10% fat) or high-fat diet (HFD, 60% fat) for 3 or 7 days. We measured skeletal muscle mitochondrial respiration and protein markers of mitophagy in a mitochondrial-enriched fraction of skeletal muscle. After 3 days of HFD, mice had lower lipid-supported oxidative phosphorylation alongside greater electron leak compared with the LFD group. After 7 days, there were no differences in mitochondrial respiration between diet groups. HFD mice had greater autophagosome formation potential (Beclin-1) and greater activation of mitochondrial autophagy receptors (Bnip3, p62) in isolated mitochondria, but no difference in downstream autophagosome (LC3II) or lysosome (Lamp1) abundance after both 3 and 7 days compared with the LFD groups. In cultured myotubes, palmitate treatment decreased mitochondrial membrane potential and hydrogen peroxide treatment increased accumulation of upstream mitophagy markers. We conclude that several days of high-fat feeding stimulated upstream activation of skeletal muscle mitophagy, potentially through lipid-induced oxidative stress, without downstream changes in respiration.
Keywords: autophagy; high-fat feeding; mitochondria; reactive oxygen species; respiration.
Copyright © 2021 Ehrlicher, Stierwalt, Newsom and Robinson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

