Bioaugmentation of Native Fungi, an Efficient Strategy for the Bioremediation of an Aged Industrially Polluted Soil With Heavy Hydrocarbons
- PMID: 33868189
- PMCID: PMC8044458
- DOI: 10.3389/fmicb.2021.626436
Bioaugmentation of Native Fungi, an Efficient Strategy for the Bioremediation of an Aged Industrially Polluted Soil With Heavy Hydrocarbons
Abstract
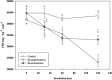
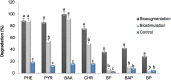
The concurrence of structurally complex petroleum-associated contaminants at relatively high concentrations, with diverse climatic conditions and textural soil characteristics, hinders conventional bioremediation processes. Recalcitrant compounds such as high molecular weight polycyclic aromatic hydrocarbons (HMW-PAHs) and heavy alkanes commonly remain after standard soil bioremediation at concentrations above regulatory limits. The present study assessed the potential of native fungal bioaugmentation as a strategy to promote the bioremediation of an aged industrially polluted soil enriched with heavy hydrocarbon fractions. Microcosms assays were performed by means of biostimulation and bioaugmentation, by inoculating a defined consortium of six potentially hydrocarbonoclastic fungi belonging to the genera Penicillium, Ulocladium, Aspergillus, and Fusarium, which were isolated previously from the polluted soil. The biodegradation performance of fungal bioaugmentation was compared with soil biostimulation (water and nutrient addition) and with untreated soil as a control. Fungal bioaugmentation resulted in a higher biodegradation of total petroleum hydrocarbons (TPH) and of HMW-PAHs than with biostimulation. TPH (C14-C35) decreased by a 39.90 ± 1.99% in bioaugmented microcosms vs. a 24.17 ± 1.31% in biostimulated microcosms. As for the effect of fungal bioaugmentation on HMW-PAHs, the 5-ringed benzo(a)fluoranthene and benzo(a)pyrene were reduced by a 36% and 46%, respectively, while the 6-ringed benzoperylene decreased by a 28%, after 120 days of treatment. Biostimulated microcosm exhibited a significantly lower reduction of 5- and 6-ringed PAHs (8% and 5% respectively). Higher TPH and HMW-PAHs biodegradation levels in bioaugmented microcosms were also associated to a significant decrease in acute ecotoxicity (EC50) by Vibrio fischeri bioluminiscence inhibition assays. Molecular profiling and counting of viable hydrocarbon-degrading bacteria from soil microcosms revealed that fungal bioaugmentation promoted the growth of autochthonous active hydrocarbon-degrading bacteria. The implementation of such an approach to enhance hydrocarbon biodegradation should be considered as a novel bioremediation strategy for the treatment of the most recalcitrant and highly genotoxic hydrocarbons in aged industrially polluted soils.
Keywords: aged-polluted soil; fungal-bacterial interactions; high molecular weight polycyclic aromatic hydrocarbons; indigenous hydrocarbonoclastic fungi; mycoremediation; native-fungal-bioaugmentation.
Copyright © 2021 Medaura, Guivernau, Moreno-Ventas, Prenafeta-Boldú and Viñas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Alexander M. (1999). Biodegradation and Bioremediation, 2nd Edn. San Diego: Academic Press.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

