Immunomodulation in Administration of rAAV: Preclinical and Clinical Adjuvant Pharmacotherapies
- PMID: 33868303
- PMCID: PMC8049138
- DOI: 10.3389/fimmu.2021.658038
Immunomodulation in Administration of rAAV: Preclinical and Clinical Adjuvant Pharmacotherapies
Abstract
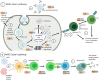
Recombinant adeno-associated virus (rAAV) has attracted a significant research focus for delivering genetic therapies to target cells. This non-enveloped virus has been trialed in many clinical-stage therapeutic strategies but important obstacle in clinical translation is the activation of both innate and adaptive immune response to the protein capsid, vector genome and transgene product. In addition, the normal population has pre-existing neutralizing antibodies against wild-type AAV, and cross-reactivity is observed between different rAAV serotypes. While extent of response can be influenced by dosing, administration route and target organ(s), these pose concerns over reduction or complete loss of efficacy, options for re-administration, and other unwanted immunological sequalae such as local tissue damage. To reduce said immunological risks, patients are excluded if they harbor anti-AAV antibodies or have received gene therapy previously. Studies have incorporated immunomodulating or suppressive regimens to block cellular and humoral immune responses such as systemic corticosteroids pre- and post-administration of Luxturna® and Zolgensma®, the two rAAV products with licensed regulatory approval in Europe and the United States. In this review, we will introduce the current pharmacological strategies to immunosuppress or immunomodulate the host immune response to rAAV gene therapy.
Keywords: adeno associated virus; gene therapy; immune response; immunomodulation; immunosuppression; pharmacotherapies.
Copyright © 2021 Chu and Ng.
Conflict of interest statement
JN has sponsored research agreements with AskBio Europe and Rocket Pharma. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates.J Transl Med. 2012 Jun 15;10:122. doi: 10.1186/1479-5876-10-122. J Transl Med. 2012. PMID: 22704060 Free PMC article.
-
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression.Int J Nanomedicine. 2024 Jul 29;19:7691-7708. doi: 10.2147/IJN.S459905. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099791 Free PMC article. Review.
-
Pre-Existing Immunity to a Nucleic Acid Contaminant-Derived Antigen Mediates Transaminitis and Resultant Diminished Transgene Expression in a Mouse Model of Hepatic Recombinant Adeno-Associated Virus-Mediated Gene Transfer.Hum Gene Ther. 2024 Jul;35(13-14):477-489. doi: 10.1089/hum.2023.188. Epub 2024 Mar 28. Hum Gene Ther. 2024. PMID: 38420654
-
Muscle-Directed Delivery of an AAV1 Vector Leads to Capsid-Specific T Cell Exhaustion in Nonhuman Primates and Humans.Mol Ther. 2020 Mar 4;28(3):747-757. doi: 10.1016/j.ymthe.2020.01.004. Epub 2020 Jan 13. Mol Ther. 2020. PMID: 31982038 Free PMC article.
-
Overcoming the Challenges Imposed by Humoral Immunity to AAV Vectors to Achieve Safe and Efficient Gene Transfer in Seropositive Patients.Front Immunol. 2022 Apr 7;13:857276. doi: 10.3389/fimmu.2022.857276. eCollection 2022. Front Immunol. 2022. PMID: 35464422 Free PMC article. Review.
Cited by
-
Gene therapy: Practical aspects of implementation.Haemophilia. 2022 May;28 Suppl 4(Suppl 4):44-52. doi: 10.1111/hae.14545. Haemophilia. 2022. PMID: 35521727 Free PMC article. Review.
-
UKHCDO gene therapy taskforce: Guidance for implementation of haemophilia gene therapy into routine clinical practice for adults.Haemophilia. 2025 Jan;31(1):26-38. doi: 10.1111/hae.15125. Epub 2024 Nov 20. Haemophilia. 2025. PMID: 39565651 Free PMC article.
-
Advancements and Innovations in the Surgical Management of Sacrococcygeal Pilonidal Sinus: A Comprehensive Review.Cureus. 2024 May 26;16(5):e61141. doi: 10.7759/cureus.61141. eCollection 2024 May. Cureus. 2024. PMID: 38933617 Free PMC article. Review.
-
Therapeutic Application of Extracellular Vesicles-Capsulated Adeno-Associated Virus Vector via nSMase2/Smpd3, Satellite, and Immune Cells in Duchenne Muscular Dystrophy.Int J Mol Sci. 2022 Jan 28;23(3):1551. doi: 10.3390/ijms23031551. Int J Mol Sci. 2022. PMID: 35163475 Free PMC article. Review.
-
FSHD Therapeutic Strategies: What Will It Take to Get to Clinic?J Pers Med. 2022 May 25;12(6):865. doi: 10.3390/jpm12060865. J Pers Med. 2022. PMID: 35743650 Free PMC article. Review.
References
-
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum Gene Ther (2010) 21:704–12. 10.1089/hum.2009.182 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

