The Diverse Roles of Heme Oxygenase-1 in Tumor Progression
- PMID: 33868304
- PMCID: PMC8044534
- DOI: 10.3389/fimmu.2021.658315
The Diverse Roles of Heme Oxygenase-1 in Tumor Progression
Abstract
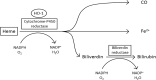
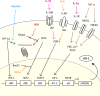
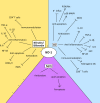
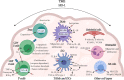
Heme oxygenase-1 (HO-1) is an inducible intracellular enzyme that is expressed in response to a variety of stimuli to degrade heme, which generates the biologically active catabolites carbon monoxide (CO), biliverdin and ferrous iron (Fe2+). HO-1 is expressed across a range of cancers and has been demonstrated to promote tumor progression through a variety of mechanisms. HO-1 can be expressed in a variety of cells within the tumor microenvironment (TME), including both the malignant tumor cells as well as stromal cell populations such as macrophages, dendritic cells and regulatory T-cells. Intrinsically to the cell, HO-1 activity provides antioxidant, anti-apoptotic and cytoprotective effects via its catabolites as well as clearing toxic intracellular heme. However, the catabolites of heme degradation can also diffuse outside of the cell to extrinsically modulate the wider TME, influencing cellular functionality and biological processes which promote tumor progression, such as facilitating angiogenesis and metastasis, as well as promoting anti-inflammation and immune suppression. Pharmacological inhibition of HO-1 has been demonstrated to be a promising therapeutic approach to promote anti-tumor immune responses and inhibit metastasis. However, these biological functions might be context, TME and cell type-dependent as there is also conflicting reports for HO-1 activity facilitating anti-tumoral processes. This review will consider our current understanding of the role of HO-1 in cancer progression and as a therapeutic target in cancer.
Keywords: angiogenesis; cancer; cytoprotection; heme oxygenase-1 (HO-1); metastasis; tumor associated macrophages (TAMs); tumor immunology.
Copyright © 2021 Luu Hoang, Anstee and Arnold.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Heme oxygenase-1 orchestrates the immunosuppressive program of tumor-associated macrophages.JCI Insight. 2020 Jun 4;5(11):e133929. doi: 10.1172/jci.insight.133929. JCI Insight. 2020. PMID: 32369450 Free PMC article.
-
Heme oxygenase-1 in tumors: is it a false friend?Antioxid Redox Signal. 2007 Dec;9(12):2099-117. doi: 10.1089/ars.2007.1659. Antioxid Redox Signal. 2007. PMID: 17822372 Free PMC article. Review.
-
Breast cancer cell debris diminishes therapeutic efficacy through heme oxygenase-1-mediated inactivation of M1-like tumor-associated macrophages.Neoplasia. 2020 Nov;22(11):606-616. doi: 10.1016/j.neo.2020.08.006. Epub 2020 Oct 8. Neoplasia. 2020. PMID: 33039895 Free PMC article.
-
Heme oxygenase-1 in tumor biology and therapy.Curr Drug Targets. 2010 Dec;11(12):1551-70. doi: 10.2174/1389450111009011551. Curr Drug Targets. 2010. PMID: 20704546 Review.
-
Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1.Free Radic Biol Med. 2014 Feb;67:353-65. doi: 10.1016/j.freeradbiomed.2013.10.819. Epub 2013 Nov 5. Free Radic Biol Med. 2014. PMID: 24200599 Review.
Cited by
-
Targeting heme degradation pathway augments prostate cancer cell sensitivity to docetaxel-induced apoptosis and attenuates migration.Front Oncol. 2024 Jul 18;14:1431362. doi: 10.3389/fonc.2024.1431362. eCollection 2024. Front Oncol. 2024. PMID: 39091910 Free PMC article.
-
Network pharmacology study and in vitro experimental validation of Xiaojianzhong decoction against gastric cancer.World J Gastrointest Oncol. 2024 Sep 15;16(9):3932-3954. doi: 10.4251/wjgo.v16.i9.3932. World J Gastrointest Oncol. 2024. PMID: 39350988 Free PMC article.
-
Transcriptomics and metabolomics reveal changes in the regulatory mechanisms of osteosarcoma under different culture methods in vitro.BMC Med Genomics. 2022 Dec 19;15(1):265. doi: 10.1186/s12920-022-01419-1. BMC Med Genomics. 2022. PMID: 36536381 Free PMC article.
-
Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways.Biomedicines. 2021 Sep 22;9(10):1295. doi: 10.3390/biomedicines9101295. Biomedicines. 2021. PMID: 34680412 Free PMC article.
-
Artificial blood-hope and the challenges to combat tumor hypoxia for anti-cancer therapy.Med Biol Eng Comput. 2025 Apr;63(4):933-957. doi: 10.1007/s11517-024-03233-6. Epub 2024 Nov 30. Med Biol Eng Comput. 2025. PMID: 39614063 Review.
References
-
- Muliaditan T, Opzoomer JW, Caron J, Okesola M, Kosti P, Lall S, et al. . Repurposing Tin Mesoporphyrin as an Immune Checkpoint Inhibitor Shows Therapeutic Efficacy in Preclinical Models of Cancer. Clin Cancer Res An Off J Am Assoc Cancer Res (2018) 24(7):1617–28. 10.1158/1078-0432.CCR-17-2587 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

