TREX1 as a Novel Immunotherapeutic Target
- PMID: 33868310
- PMCID: PMC8047136
- DOI: 10.3389/fimmu.2021.660184
TREX1 as a Novel Immunotherapeutic Target
Abstract
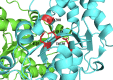
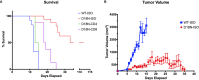
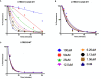
Mutations in the TREX1 3' → 5' exonuclease are associated with a spectrum of autoimmune disease phenotypes in humans and mice. Failure to degrade DNA activates the cGAS-STING DNA-sensing pathway signaling a type-I interferon (IFN) response that ultimately drives immune system activation. TREX1 and the cGAS-STING DNA-sensing pathway have also been implicated in the tumor microenvironment, where TREX1 is proposed to degrade tumor-derived DNA that would otherwise activate cGAS-STING. If tumor-derived DNA were not degraded, the cGAS-STING pathway would be activated to promote IFN-dependent antitumor immunity. Thus, we hypothesize TREX1 exonuclease inhibition as a novel immunotherapeutic strategy. We present data demonstrating antitumor immunity in the TREX1 D18N mouse model and discuss theory surrounding the best strategy for TREX1 inhibition. Potential complications of TREX1 inhibition as a therapeutic strategy are also discussed.
Keywords: cancer; exonuclease; immunotherapy; inhibition; small-molecule.
Copyright © 2021 Hemphill, Simpson, Liu, Salsbury, Hollis, Grayson and Perrino.
Conflict of interest statement
WH, TH, and FP declare the filing of U.S. Provisional Application No. 62/706,167 Trex1 Inhibitors and Uses Thereof. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


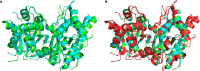

Similar articles
-
The induction of type I interferonopathy in Trex1-P212fs mice is mediated by activation of the cGAS-STING pathway.Int J Biol Macromol. 2025 May;310(Pt 2):143414. doi: 10.1016/j.ijbiomac.2025.143414. Epub 2025 Apr 21. Int J Biol Macromol. 2025. PMID: 40268028
-
Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases.Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):E5699-705. doi: 10.1073/pnas.1516465112. Epub 2015 Sep 14. Proc Natl Acad Sci U S A. 2015. PMID: 26371324 Free PMC article.
-
Cutting Edge: cGAS Is Required for Lethal Autoimmune Disease in the Trex1-Deficient Mouse Model of Aicardi-Goutières Syndrome.J Immunol. 2015 Sep 1;195(5):1939-43. doi: 10.4049/jimmunol.1500969. Epub 2015 Jul 29. J Immunol. 2015. PMID: 26223655 Free PMC article.
-
TREX1 plays multiple roles in human diseases.Cell Immunol. 2022 May;375:104527. doi: 10.1016/j.cellimm.2022.104527. Epub 2022 Apr 21. Cell Immunol. 2022. PMID: 35468328 Review.
-
An emerging role for calcium signalling in innate and autoimmunity via the cGAS-STING axis.Cytokine Growth Factor Rev. 2019 Dec;50:43-51. doi: 10.1016/j.cytogfr.2019.04.003. Epub 2019 Apr 2. Cytokine Growth Factor Rev. 2019. PMID: 30955997 Review.
Cited by
-
Interplay between the DNA Damage Response and Immunotherapy Response in Cancer.Int J Mol Sci. 2022 Nov 1;23(21):13356. doi: 10.3390/ijms232113356. Int J Mol Sci. 2022. PMID: 36362142 Free PMC article. Review.
-
Radiotherapy induced immunogenic cell death by remodeling tumor immune microenvironment.Front Immunol. 2022 Dec 1;13:1074477. doi: 10.3389/fimmu.2022.1074477. eCollection 2022. Front Immunol. 2022. PMID: 36532071 Free PMC article. Review.
-
Structural basis of human TREX1 DNA degradation and autoimmune disease.Nat Commun. 2022 Jul 25;13(1):4277. doi: 10.1038/s41467-022-32055-z. Nat Commun. 2022. PMID: 35879334 Free PMC article.
-
From mechanism to therapy: the journey of CD24 in cancer.Front Immunol. 2024 May 31;15:1401528. doi: 10.3389/fimmu.2024.1401528. eCollection 2024. Front Immunol. 2024. PMID: 38881902 Free PMC article. Review.
-
Multiple RNA- and DNA-binding proteins exhibit direct transfer of polynucleotides with implications for target-site search.Proc Natl Acad Sci U S A. 2023 Jun 27;120(26):e2220537120. doi: 10.1073/pnas.2220537120. Epub 2023 Jun 20. Proc Natl Acad Sci U S A. 2023. PMID: 37339225 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

