A Meloidogyne graminicola Pectate Lyase Is Involved in Virulence and Activation of Host Defense Responses
- PMID: 33868351
- PMCID: PMC8044864
- DOI: 10.3389/fpls.2021.651627
A Meloidogyne graminicola Pectate Lyase Is Involved in Virulence and Activation of Host Defense Responses
Abstract
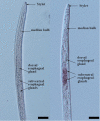
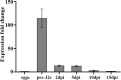
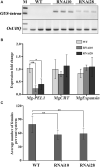
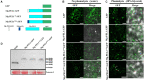
Plant-parasitic nematodes secrete an array of cell-wall-degrading enzymes to overcome the physical barrier formed by the plant cell wall. Here, we describe a novel pectate lyase gene Mg-PEL1 from M. graminicola. Quantitative real-time PCR assay showed that the highest transcriptional expression level of Mg-PEL1 occurred in pre-parasitic second-stage juveniles, and it was still detected during the early parasitic stage. Using in situ hybridization, we showed that Mg-PEL1 was expressed exclusively within the subventral esophageal gland cells of M. graminicola. The yeast signal sequence trap system revealed that it possessed an N-terminal signal peptide with secretion function. Recombinant Mg-PEL1 exhibited hydrolytic activity toward polygalacturonic acid. Rice plants expressing RNA interference vectors targeting Mg-PEL1 showed an increased resistance to M. graminicola. In addition, using an Agrobacterium-mediated transient expression system and plant immune response assays, we demonstrated that the cell wall localization of Mg-PEL1 was required for the activation of plant defense responses, including programmed plant cell death, reactive oxygen species (ROS) accumulation and expression of defense-related genes. Taken together, our results indicated that Mg-PEL1 could enhance the pathogenicity of M. graminicola and induce plant immune responses during nematode invasion into plants or migration in plants. This provides a new insight into the function of pectate lyases in plants-nematodes interaction.
Keywords: Meloidogyne graminicola; RNAi; enzymatic activity; pectate lyase; plant immunity elicitation.
Copyright © 2021 Chen, Li, Lin, Liao and Zhuo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Charkowski A. O., Alfano J. R., Preston G., Yuan J., He S. Y., Collmer A. (1998). The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J. Bacteriol. 180 5211–5217. 10.1128/JB.180.19.5211-5217.1998 - DOI - PMC - PubMed
-
- Chen J., Lin B., Huang Q., Hu L., Zhuo K., Liao J. (2017). A novel Meloidogyne graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism. PLoS Pathog. 13:e1006301. 10.1371/journal.ppat.1006301 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

