Targeting HIF-α for robust prevascularization of human cardiac organoids
- PMID: 33868541
- PMCID: PMC8049092
- DOI: 10.1002/term.3165
Targeting HIF-α for robust prevascularization of human cardiac organoids
Abstract
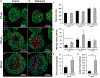
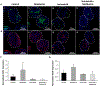
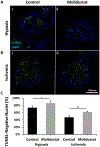
Prevascularized 3D microtissues have been shown to be an effective cell delivery vehicle for cardiac repair. To this end, our lab has explored the development of self-organizing, prevascularized human cardiac organoids by co-seeding human cardiomyocytes with cardiac fibroblasts, endothelial cells, and stromal cells into agarose microwells. We hypothesized that this prevascularization process is facilitated by the endogenous upregulation of hypoxia-inducible factor (HIF) pathway in the avascular 3D microtissues. In this study, we used Molidustat, a selective PHD (prolyl hydroxylase domain enzymes) inhibitor that stabilizes HIF-α, to treat human cardiac organoids, which resulted in 150 ± 61% improvement in endothelial expression (CD31) and 220 ± 20% improvement in the number of lumens per organoids. We hypothesized that the improved endothelial expression seen in Molidustat treated human cardiac organoids was dependent upon upregulation of VEGF, a well-known downstream target of HIF pathway. Through the use of immunofluorescent staining and ELISA assays, we determined that Molidustat treatment improved VEGF expression of non-endothelial cells and resulted in improved co-localization of supporting cell types and endothelial structures. We further demonstrated that Molidustat treated human cardiac organoids maintain cardiac functionality. Lastly, we showed that Molidustat treatment improves survival of cardiac organoids when exposed to both hypoxic and ischemic conditions in vitro. For the first time, we demonstrate that targeted HIF-α stabilization provides a robust strategy to improve endothelial expression and lumen formation in cardiac microtissues, which will provide a powerful framework for prevascularization of various microtissues in developing successful cell transplantation therapies.
Keywords: HIF; HIF prolyl-hydroxylase inhibitor; human cardiac organoid; human induced stem cell-derived cardiomyocyte; prevascularization; tissue engineering.
Conflict of interest statement
Conflicts of Interest Statement The authors have no conflicts of interest to report.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

