Ataxia Telangiectasia Mutated Protein Kinase: A Potential Master Puppeteer of Oxidative Stress-Induced Metabolic Recycling
- PMID: 33868575
- PMCID: PMC8032526
- DOI: 10.1155/2021/8850708
Ataxia Telangiectasia Mutated Protein Kinase: A Potential Master Puppeteer of Oxidative Stress-Induced Metabolic Recycling
Abstract
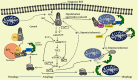
Ataxia Telangiectasia Mutated protein kinase (ATM) has recently come to the fore as a regulatory protein fulfilling many roles in the fine balancing act of metabolic homeostasis. Best known for its role as a transducer of DNA damage repair, the activity of ATM in the cytosol is enjoying increasing attention, where it plays a central role in general cellular recycling (macroautophagy) as well as the targeted clearance (selective autophagy) of damaged mitochondria and peroxisomes in response to oxidative stress, independently of the DNA damage response. The importance of ATM activation by oxidative stress has also recently been highlighted in the clearance of protein aggregates, where the expression of a functional ATM construct that cannot be activated by oxidative stress resulted in widespread accumulation of protein aggregates. This review will discuss the role of ATM in general autophagy, mitophagy, and pexophagy as well as aggrephagy and crosstalk between oxidative stress as an activator of ATM and its potential role as a master regulator of these processes.
Copyright © 2021 Marguerite Blignaut et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Neurodegeneration in ataxia-telangiectasia: Multiple roles of ATM kinase in cellular homeostasis.Dev Dyn. 2018 Jan;247(1):33-46. doi: 10.1002/dvdy.24522. Epub 2017 Jun 5. Dev Dyn. 2018. PMID: 28543935 Review.
-
Oxidative stress and the multifaceted roles of ATM in maintaining cellular redox homeostasis.Redox Biol. 2024 Sep;75:103269. doi: 10.1016/j.redox.2024.103269. Epub 2024 Jul 16. Redox Biol. 2024. PMID: 39018798 Free PMC article. Review.
-
Activation of ATM kinase by ROS generated during ionophore-induced mitophagy in human T and B cell malignancies.Mol Cell Biochem. 2021 Jan;476(1):417-423. doi: 10.1007/s11010-020-03917-1. Epub 2020 Sep 29. Mol Cell Biochem. 2021. PMID: 32996079 Free PMC article.
-
Mechanisms of ATM Activation.Annu Rev Biochem. 2015;84:711-38. doi: 10.1146/annurev-biochem-060614-034335. Epub 2015 Jan 12. Annu Rev Biochem. 2015. PMID: 25580527 Review.
-
Recently emerging signaling landscape of ataxia-telangiectasia mutated (ATM) kinase.Asian Pac J Cancer Prev. 2014;15(16):6485-8. doi: 10.7314/apjcp.2014.15.16.6485. Asian Pac J Cancer Prev. 2014. PMID: 25169474 Review.
Cited by
-
Discovery of Therapeutics Targeting Oxidative Stress in Autosomal Recessive Cerebellar Ataxia: A Systematic Review.Pharmaceuticals (Basel). 2022 Jun 19;15(6):764. doi: 10.3390/ph15060764. Pharmaceuticals (Basel). 2022. PMID: 35745683 Free PMC article. Review.
-
Phenotypic and Genetic Complexity in Pediatric Movement Disorders.Front Genet. 2022 Jun 1;13:829558. doi: 10.3389/fgene.2022.829558. eCollection 2022. Front Genet. 2022. PMID: 35719373 Free PMC article.
-
Ataxia-telangiectasia clinical trial landscape and the obstacles to overcome.Expert Opin Investig Drugs. 2023 Jul-Dec;32(8):693-704. doi: 10.1080/13543784.2023.2249399. Epub 2023 Aug 28. Expert Opin Investig Drugs. 2023. PMID: 37622329 Free PMC article. Review.
-
Ataxia-telangiectasia mutated plays an important role in cerebellar integrity and functionality.Neural Regen Res. 2023 Mar;18(3):497-502. doi: 10.4103/1673-5374.350194. Neural Regen Res. 2023. PMID: 36018153 Free PMC article. Review.
-
Disproportionate Expression of ATM in Cerebellar Cortex During Human Neurodevelopment.Cerebellum. 2024 Apr;23(2):502-511. doi: 10.1007/s12311-023-01560-2. Epub 2023 Apr 29. Cerebellum. 2024. PMID: 37120494 Free PMC article.
References
-
- Syllaba L., Henner K. Contribution a l’independance de l’athetose double idiopathique et congenitale. Revista de Neurologia. 1926;1:541–562.
-
- Boder E., Sedgwick R. P. Ataxia-telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics. 1958;21(4):526–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

