Reconstruction with customized, 3D-printed prosthesis after resection of periacetabular Ewing's sarcoma in children using "triradiate cartilage-based" surgical strategy:a technical note
- PMID: 33868923
- PMCID: PMC8022806
- DOI: 10.1016/j.jot.2020.12.006
Reconstruction with customized, 3D-printed prosthesis after resection of periacetabular Ewing's sarcoma in children using "triradiate cartilage-based" surgical strategy:a technical note
Abstract
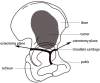
Background: Surgery for Ewing sarcoma involving acetabulum in children is challenging. Considering the intrinsic structure of immature pelvis, trans-acetabular osteotomy through triradiate cartilage might be applied. The study was to describe the surgical technique and function outcomes of trans-acetabular osteotomy through triradiate cartilage and reconstruction with customized, 3D-printed prosthesis.
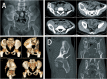
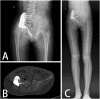
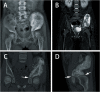
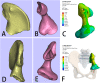
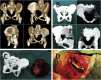
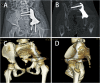
Methods: Two children with periacetabular ES were admitted to our hospital. The pre-operative imaging showed the triradiate cartilage was not penetrated or wholly affected by tumor. After neoadjuvant chemotherapy, the tumor was excised by trans-acetabular osteotomy basing on "triradiate cartilage strategy" and the acetabulum was reconstructed with the customized, 3D-printed prosthesis. The prosthesis was designed in Mimics software basing on the images from CT, optimized by topology technique, and examined in FE model. After implantation, the oncological and functional outcomes were evaluated with radiography, CT, and MSTS score.
Results: The operation time and intra-operative blood loss in these two children were 3.5h, 2.5h and 300 ml, 600 ml, respectively. The postoperative specimen showed the tumor was en bloc removed with safe margin. In the latest follow-up (48 months and 24 months), both patients were free of disease and had satisfactory function according to MSTS score. The radiography indicated the prosthesis fit the defect well without loosening.
Conclusion: The customized, 3D-printed prosthesis could provide optimal reconstruction of pelvic ring and satisfactory hip function after trans-acetabular osteotomy in children.
The translational potential of this article: This study provides promising results of implantation of customized 3D printing prosthesis in children's pelvic sarcoma, which may bring a new design method for orthopaedic implants.
Keywords: 3D printing; Acetabulum; Ewing sarcoma; Pelvis; Prosthesis.
© 2021 The Authors.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Figures


Similar articles
-
Individual resection and reconstruction of pelvic tumor with three-dimensional printed customized hemi-pelvic prosthesis: A case report.Medicine (Baltimore). 2019 Sep;98(36):e16658. doi: 10.1097/MD.0000000000016658. Medicine (Baltimore). 2019. PMID: 31490360 Free PMC article.
-
Biomechanical and clinical outcomes of 3D-printed versus modular hemipelvic prostheses for limb-salvage reconstruction following periacetabular tumor resection: a mid-term retrospective cohort study.J Orthop Surg Res. 2024 Apr 23;19(1):258. doi: 10.1186/s13018-024-04697-w. J Orthop Surg Res. 2024. PMID: 38654343 Free PMC article.
-
Ewing's sarcoma of proximal femur: case report of extreme osteotomy with 3D-printed prosthesis for the reconstruction.Front Bioeng Biotechnol. 2023 Oct 9;11:1248330. doi: 10.3389/fbioe.2023.1248330. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37877038 Free PMC article.
-
Pelvic fractures in children (pelvic ring and acetabulum).Orthop Traumatol Surg Res. 2020 Feb;106(1S):S125-S133. doi: 10.1016/j.otsr.2019.05.017. Epub 2019 Sep 11. Orthop Traumatol Surg Res. 2020. PMID: 31521559 Review.
-
Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: a case series and review of the literature.World J Surg Oncol. 2015 Nov 4;13:308. doi: 10.1186/s12957-015-0723-2. World J Surg Oncol. 2015. PMID: 26537339 Free PMC article. Review.
Cited by
-
Effect of carbon-fiber-reinforced polyetheretherketone on stress distribution in a redesigned tumor-type knee prosthesis: a finite element analysis.Front Bioeng Biotechnol. 2023 Sep 26;11:1243936. doi: 10.3389/fbioe.2023.1243936. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37823023 Free PMC article.
-
Computer-Aided Design and 3D Printing of Hemipelvic Endoprosthesis for Personalized Limb-Salvage Reconstruction after Periacetabular Tumor Resection.Bioengineering (Basel). 2022 Aug 18;9(8):400. doi: 10.3390/bioengineering9080400. Bioengineering (Basel). 2022. PMID: 36004925 Free PMC article.
-
[Pelvic limb-salvage surgery for malignant tumors: 30 years of progress in China].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022 Jul 15;36(7):781-789. doi: 10.7507/1002-1892.202112059. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022. PMID: 35848171 Free PMC article. Review. Chinese.
-
Clinical Effect of Laparoscopic Radical Surgery Combined with Neoadjuvant Chemotherapy in Treating Cervical Cancer and Its Influence on Postoperative Complications and Adverse Reaction Rates.J Healthc Eng. 2022 Feb 9;2022:8768188. doi: 10.1155/2022/8768188. eCollection 2022. J Healthc Eng. 2022. PMID: 35186243 Free PMC article.
-
Exploring the optimal reconstruction strategy for Enneking III defects in pelvis bone tumors: a finite element analysis.J Orthop Surg Res. 2025 Jan 24;20(1):96. doi: 10.1186/s13018-025-05500-0. J Orthop Surg Res. 2025. PMID: 39856781 Free PMC article.
References
-
- Verma N.N., Kuo K.N., Gitelis S. Acetabular osteoarticular allograft after Ewing’s sarcoma resection. Clin Orthop Relat Res. 2004;(419):149–154. - PubMed
-
- Obata H., Ueda T., Kawai A., Ishii T., Ozaki T., Abe S. Clinical outcome of patients with Ewing sarcoma family of tumors of bone in Japan: the Japanese Musculoskeletal Oncology Group cooperative study. Cancer. 2007;109(4):767–775. - PubMed
-
- Frassica F.J., Frassica D.A., Pritchard D.J., Schomberg P.J., Wold L.E., Sim F.H. Ewing sarcoma of the pelvis. Clinicopathological features and treatment. J Bone Joint Surg Am. 1993;75(10):1457–1465. - PubMed
-
- Enneking W.F., Dunham W.K. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60(6):731–746. - PubMed
-
- Fujiwara T., Lex J.R., Stevenson J.D., Tsuda Y., Clark R., Parry M.C. Surgical treatment for pelvic Ewing sarcoma: what is a safe and functional acetabular reconstruction when combined with modern multidisciplinary treatments? J Surg Oncol. 2019;120(6):985–993. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

