Characterizing the Genomic Landscape of Brain Glioma With Circulating Tumor DNA From Tumor In Situ Fluid
- PMID: 33868989
- PMCID: PMC8045748
- DOI: 10.3389/fonc.2021.584988
Characterizing the Genomic Landscape of Brain Glioma With Circulating Tumor DNA From Tumor In Situ Fluid
Abstract
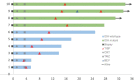
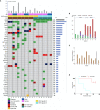
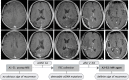
Tumor in situ fluid (TISF) refers to the fluid at the local surgical cavity. We evaluated the feasibility of TISF-derived circulating tumor DNA (ctDNA) characterizing the genomic landscape for glioma. This retrospective study included TISF and tumor samples from 10 patients with glioma, we extracted cell-free DNA (cfDNA) from the TISF and then performed deep sequencing on that. And we compared genomic alterations between TISF and tumor tissue. Results showed that the concentration of cfDNA fragments from the patients for TISF ranged from 7.2 to 1,397 ng/ml. At least one tumor-specific mutation was identified in all 10 patients (100%). Further analysis of TISF ctDNA revealed a broad spectrum of genetic mutations, which have been reported to have clinical relevance. The analysis of concordance between TISF and tumor tissue reflected the spatiotemporal heterogeneity of glioma. Collectively, TISF ctDNA was a powerfully potential source for characterizing the genomic landscape of glioma, which provided new possibilities for precision medicine in patients with glioma.
Keywords: circulating tumor DNA; glioma; liquid biopsy; precision medicine; tumor in situ fluid.
Copyright © 2021 Sheng, Yu, Deng, Andrade-Barazarte, Zemmar, Li, Li, Yan, Chen, Sun, Hernesniemi and Bu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Nicholson JG, Fine HA. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discovery (2021) 11(3):575–90. 10.1158/2159-8290.CD-20-1474 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

