Efficacy of First Line Systemic Chemotherapy and Multikinase Inhibitors in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-Analysis
- PMID: 33869060
- PMCID: PMC8044881
- DOI: 10.3389/fonc.2021.654020
Efficacy of First Line Systemic Chemotherapy and Multikinase Inhibitors in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-Analysis
Abstract
Background: Hepatocellular carcinoma (HCC) is the third most fatal cancer, with a 5-year survival rate of 18%. Standard frontline-therapy is multikinase inhibitors (MKIs), but accessibility is still limited, particularly in developing countries. This network meta-analysis (NMA) aimed to compare the efficacy of usual chemotherapy vs MKIs.
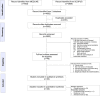
Method: Randomised-controlled trials (RCTs) comparing any among chemotherapy vs MKIs in treatment-naïve patients with advanced HCCs were identified from MEDLINE and SCOPUS databases. Overall survival (OS) and progression-free survival (PFS) probabilities and times were extracted from Kaplan-Meier curves using Digitizer, and then converted to individual patient time-to-event data. A one-stage mixed-effect survival model was applied to estimate median OS and PFS. A two-stage NMA was applied for the overall response rate and adverse events (AEs) outcome.
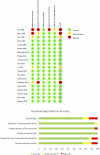
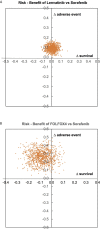
Results: A total of 20 RCTs were eligible for NMA. Lenvatinib was the best treatment among single MKIs, with median OS and PFS of 9 and 6.3 months, without significant differences in AEs relative to other MKIs. Median OS and PFS were 0.70 (-0.42, 1.83) and 2.17 (1.41, 2.93) months longer with Lenvatinib than Sorafenib. Among chemotherapy agents, FOLFOX4 had the longest median OS and PFS at 7.9 and 4.3 months, respectively, without significant AEs compared to other chemotherapies. The combination of Sorafenib+Doxorubicin prolonged median OS and PFS to 12.7 and 6.3 months, respectively.
Conclusion: Use of the MKIs Lenvatinib or Sorafenib as first line systemic treatment for advanced HCC could be beneficial. However, FOLFOX4 might be the optimal choice in a developing country where the health-care budget is limited.
Keywords: chemotherapy; first-line systemic treatment; hepatocellular carcinoma; multikinase inhibitors; network meta-analysis.
Copyright © 2021 Oranratnachai, Rattanasiri, Pooprasert, Tansawet, Reungwetwattana, Attia and Thakkinstian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Clinical Outcomes with Multikinase Inhibitors after Progression on First-Line Atezolizumab plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma: A Multinational Multicenter Retrospective Study.Liver Cancer. 2021 Apr;10(2):107-114. doi: 10.1159/000512781. Epub 2021 Mar 3. Liver Cancer. 2021. PMID: 33977087 Free PMC article.
-
Balancing Efficacy and Tolerability of First-Line Systemic Therapies for Advanced Hepatocellular Carcinoma: A Network Meta-Analysis.Liver Cancer. 2023 Jul 25;13(2):169-180. doi: 10.1159/000531744. eCollection 2024 Apr. Liver Cancer. 2023. PMID: 38751554 Free PMC article.
-
Progression-Free Survival Early Assessment Is a Robust Surrogate Endpoint of Overall Survival in Immunotherapy Trials of Hepatocellular Carcinoma.Cancers (Basel). 2020 Dec 30;13(1):90. doi: 10.3390/cancers13010090. Cancers (Basel). 2020. PMID: 33396833 Free PMC article.
-
Efficacy and safety of lenvatinib versus sorafenib in first-line treatment of advanced hepatocellular carcinoma: A meta-analysis.Front Oncol. 2022 Dec 22;12:1010726. doi: 10.3389/fonc.2022.1010726. eCollection 2022. Front Oncol. 2022. PMID: 36620586 Free PMC article.
-
First-Line Systemic Treatment Strategies for Unresectable Hepatocellular Carcinoma: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials.Front Oncol. 2021 Dec 24;11:771045. doi: 10.3389/fonc.2021.771045. eCollection 2021. Front Oncol. 2021. PMID: 35004289 Free PMC article.
Cited by
-
L-arginine combination with 5-fluorouracil inhibit hepatocellular carcinoma cells through suppressing iNOS/NO/AKT-mediated glycolysis.Front Pharmacol. 2024 May 22;15:1391636. doi: 10.3389/fphar.2024.1391636. eCollection 2024. Front Pharmacol. 2024. PMID: 38841361 Free PMC article.
-
Advancements in Hepatocellular Carcinoma: Potential Preclinical Drugs and their Future.Curr Pharm Des. 2023;29(1):2-14. doi: 10.2174/1381612829666221216114350. Curr Pharm Des. 2023. PMID: 36529919 Review.
-
Dose Consideration of Lenvatinib's Anti-Cancer Effect on Hepatocellular Carcinoma and the Potential Benefit of Combined Colchicine Therapy.Cancers (Basel). 2023 Oct 22;15(20):5097. doi: 10.3390/cancers15205097. Cancers (Basel). 2023. PMID: 37894463 Free PMC article.
References
-
- Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. . Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol (2013) 31(28):3517–24. 10.1200/jco.2012.48.4410 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

