Screening for Mycobacterium tuberculosis Infection Using Beijing/K Strain-Specific Peptides in a School Outbreak Cohort
- PMID: 33869073
- PMCID: PMC8044942
- DOI: 10.3389/fcimb.2021.599386
Screening for Mycobacterium tuberculosis Infection Using Beijing/K Strain-Specific Peptides in a School Outbreak Cohort
Abstract
Background: The Beijing strain of Mycobacterium tuberculosis (M. tb) has been most frequently isolated from TB patients in South Korea, and the hyper-virulent Beijing/K genotype is associated with TB outbreaks. To examine the diagnostic potential of Beijing/K-specific peptides, we performed IFN-γ release assays (IGRA) using a MTBK antigen tube containing Beijing/K MTBK_24800, ESAT-6, and CFP-10 peptides in a cohort studied during a school TB outbreak.
Methods: A total of 758 contacts were investigated for M. tb infection, and 43 contacts with latent TB infection (LTBI) and 25 active TB patients were enrolled based on serial screening with QuantiFERON-TB Gold In-Tube tests followed by clinical examinations. Blood collected in MTBK antigen tubes was utilized for IGRA and multiplex cytokine bead arrays. Immune responses were retested in 24 patients after TB treatment, and disease progression was investigated in subjects with LTBI.
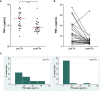
Results: Total proportions of active disease and LTBI during the outbreak were 3.7% (28/758) and 9.2% (70/758), respectively. All clinical isolates had a Beijing/K M. tb genotype. IFN-γ responses to the MTBK antigen identified M. tb infection and distinguished between active disease and LTBI. After anti-TB treatment, IFN-γ responses to the MTBK antigen were significantly reduced, and strong TNF-α responses at diagnosis were dramatically decreased.
Conclusions: MTBK antigen-specific IFN-γ has diagnostic potential for differentiating M. tb infection from healthy controls, and between active TB and LTBI as well. In addition, TNF-α is a promising marker for monitoring therapeutic responses. These data provide informative readouts for TB diagnostics and vaccine studies in regions where the Beijing/K strain is endemic.
Keywords: Beijing/K strain; IFN-γ release assay; Mycobacterium tuberculosis; cytokine; latent tuberculosis infection; outbreak.
Copyright © 2021 Hong, Kim, Park, Cho, Dockrell and Hur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



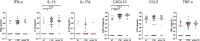

References
-
- Aagaard C. S., Hoang T. T. K. T., Vingsbo-Lundberg C., Dietrich J., Andersen P. (2009). Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J. Immunol. 183 (4), 2659–2668. 10.4049/jimmunol.0900947 - DOI - PubMed
-
- Arend S. M., Geluk A., van Meijgaarden K. E., van Dissel J. T., Theisen M., Andersen P., et al. (2000). Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68 (6), 3314–3321. 10.1128/iai.68.6.3314-3321.2000 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

