Production of Biopolyamide Precursors 5-Amino Valeric Acid and Putrescine From Rice Straw Hydrolysate by Engineered Corynebacterium glutamicum
- PMID: 33869152
- PMCID: PMC8044859
- DOI: 10.3389/fbioe.2021.635509
Production of Biopolyamide Precursors 5-Amino Valeric Acid and Putrescine From Rice Straw Hydrolysate by Engineered Corynebacterium glutamicum
Erratum in
-
Corrigendum: Production of biopolyamide precursors 5-amino valeric acid and putrescine from rice straw hydrolysate by engineered Corynebacterium glutamicum.Front Bioeng Biotechnol. 2025 Mar 24;13:1588115. doi: 10.3389/fbioe.2025.1588115. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40196156 Free PMC article.
Abstract
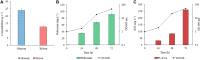
The non-proteinogenic amino acid 5-amino valeric acid (5-AVA) and the diamine putrescine are potential building blocks in the bio-polyamide industry. The production of 5-AVA and putrescine using engineered Corynebacterium glutamicum by the co-consumption of biomass-derived sugars is an attractive strategy and an alternative to their petrochemical synthesis. In our previous work, 5-AVA production from pure xylose by C. glutamicum was shown by heterologously expressing xylA from Xanthomonas campestris and xylB from C. glutamicum. Apart from this AVA Xyl culture, the heterologous expression of xylA Xc and xylB Cg was also carried out in a putrescine producing C. glutamicum to engineer a PUT Xyl strain. Even though, the pure glucose (40 g L-1) gave the maximum product yield by both the strains, the utilization of varying combinations of pure xylose and glucose by AVA Xyl and PUT Xyl in CGXII synthetic medium was initially validated. A blend of 25 g L-1 of glucose and 15 g L-1 of xylose in CGXII medium yielded 109 ± 2 mg L-1 putrescine and 874 ± 1 mg L-1 5-AVA after 72 h of fermentation. Subsequently, to demonstrate the utilization of biomass-derived sugars, the alkali (NaOH) pretreated-enzyme hydrolyzed rice straw containing a mixture of glucose (23.7 g L-1) and xylose (13.6 g L-1) was fermented by PUT Xyl and AVA Xyl to yield 91 ± 3 mg L-1 putrescine and 260 ± 2 mg L-1 5-AVA, respectively, after 72 h of fermentation. To the best of our knowledge, this is the first proof of concept report on the production of 5-AVA and putrescine using rice straw hydrolysate (RSH) as the raw material.
Keywords: 5-amino valeric acid; Corynebacterium glutamicum; polyamides; putrescine; rice straw hydrolysate.
Copyright © 2021 Sasikumar, Hannibal, Wendisch and Nampoothiri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Duff S. J. B., Murray W. D. (1996). Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour. Technol. 55 1–33. 10.1016/0960-8524(95)00122-0 - DOI
-
- Eggeling L., Bott M. (eds). (2005). Handbook of Corynebacterium glutamicum, 1st Edn, FL, United States: CRC Press. 10.1201/9781420039696 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

