Pkd2 Affects Cilia Length and Impacts LR Flow Dynamics and Dand5
- PMID: 33869175
- PMCID: PMC8047213
- DOI: 10.3389/fcell.2021.624531
Pkd2 Affects Cilia Length and Impacts LR Flow Dynamics and Dand5
Abstract
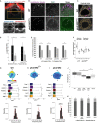
The left-right (LR) field recognizes the importance of the mechanism involving the calcium permeable channel Polycystin-2. However, whether the early LR symmetry breaking mechanism is exclusively via Polycystin-2 has not been tested. For that purpose, we need to be able to isolate the effects of decreasing the levels of Pkd2 protein from any eventual effects on flow dynamics. Here we demonstrate that curly-up (cup) homozygous mutants have abnormal flow dynamics. In addition, we performed one cell stage Pkd2 knockdowns and LR organizer specific Pkd2 knockdowns and observed that both techniques resulted in shorter cilia length and abnormal flow dynamics. We conclude that Pkd2 reduction leads to LR defects that cannot be assigned exclusively to its putative role in mediating mechanosensation because indirectly, by modifying cell shape or decreasing cilia length, Pkd2 deficit affects LR flow dynamics.
Keywords: Pkd2; cilia length; dand5; flow dynamics; left-right; zebrafish.
Copyright © 2021 Jacinto, Sampaio, Roxo-Rosa, Pestana and Lopes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis.Dev Biol. 2005 Nov 15;287(2):274-88. doi: 10.1016/j.ydbio.2005.08.047. Epub 2005 Oct 7. Dev Biol. 2005. PMID: 16216239
-
Bicc1 and Dicer regulate left-right patterning through post-transcriptional control of the Nodal inhibitor Dand5.Nat Commun. 2021 Sep 16;12(1):5482. doi: 10.1038/s41467-021-25464-z. Nat Commun. 2021. PMID: 34531379 Free PMC article.
-
An Early Function of Polycystin-2 for Left-Right Organizer Induction in Xenopus.iScience. 2018 Apr 27;2:76-85. doi: 10.1016/j.isci.2018.03.011. Epub 2018 Apr 7. iScience. 2018. PMID: 30428378 Free PMC article.
-
Function of nodal cilia in left-right determination: Mechanical regulation in initiation of symmetry breaking.Biophys Physicobiol. 2024 Sep 6;21(3):e210018. doi: 10.2142/biophysico.bppb-v21.0018. eCollection 2024. Biophys Physicobiol. 2024. PMID: 39802743 Free PMC article. Review.
-
Cilia multifunctional organelles at the center of vertebrate left-right asymmetry.Curr Top Dev Biol. 2008;85:151-74. doi: 10.1016/S0070-2153(08)00806-5. Curr Top Dev Biol. 2008. PMID: 19147005 Review.
Cited by
-
Sequential action of JNK genes establishes the embryonic left-right axis.Development. 2022 May 1;149(9):dev200136. doi: 10.1242/dev.200136. Epub 2022 May 3. Development. 2022. PMID: 35352808 Free PMC article.
-
Breaking Left-Right Symmetry by the Interplay of Planar Cell Polarity, Calcium Signaling and Cilia.Cells. 2024 Dec 20;13(24):2116. doi: 10.3390/cells13242116. Cells. 2024. PMID: 39768206 Free PMC article. Review.
-
Understanding laterality disorders and the left-right organizer: Insights from zebrafish.Front Cell Dev Biol. 2022 Dec 23;10:1035513. doi: 10.3389/fcell.2022.1035513. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36619867 Free PMC article. Review.
-
Fluid extraction from the left-right organizer uncovers mechanical properties needed for symmetry breaking.Elife. 2023 Jul 21;12:e83861. doi: 10.7554/eLife.83861. Elife. 2023. PMID: 37477290 Free PMC article.
-
Zebrafish Model as a Screen to Prevent Cyst Inflation in Autosomal Dominant Polycystic Kidney Disease.Int J Mol Sci. 2021 Aug 20;22(16):9013. doi: 10.3390/ijms22169013. Int J Mol Sci. 2021. PMID: 34445719 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

