Extracellular Vesicles in Tumors: A Potential Mediator of Bone Metastasis
- PMID: 33869189
- PMCID: PMC8047145
- DOI: 10.3389/fcell.2021.639514
Extracellular Vesicles in Tumors: A Potential Mediator of Bone Metastasis
Abstract
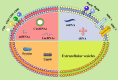
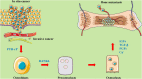
As one of the most common metastatic sites, bone has a unique microenvironment for the growth and prosperity of metastatic tumor cells. Bone metastasis is a common complication for tumor patients and accounts for 15-20% of systemic metastasis, which is only secondary to lung and liver metastasis. Cancers prone to bone metastasis include lung, breast, and prostate cancer. Extracellular vesicles (EVs) are lipid membrane vesicles released from different cell types. It is clear that EVs are associated with multiple biological phenomena and are crucial for intracellular communication by transporting intracellular substances. Recent studies have implicated EVs in the development of cancer. However, the potential roles of EVs in the pathological exchange of bone cells between tumors and the bone microenvironment remain an emerging area. This review is focused on the role of tumor-derived EVs in bone metastasis and possible regulatory mechanisms.
Keywords: bone metastasis; extracellular vesicles; osteoblast; osteoclast; tumor.
Copyright © 2021 Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Extracellular Vesicle-Mediated Bone Remodeling and Bone Metastasis: Implications in Prostate Cancer.Subcell Biochem. 2021;97:297-361. doi: 10.1007/978-3-030-67171-6_12. Subcell Biochem. 2021. PMID: 33779922 Review.
-
Restoring Tissue Homeostasis at Metastatic Sites: A Focus on Extracellular Vesicles in Bone Metastasis.Front Oncol. 2021 Mar 22;11:644109. doi: 10.3389/fonc.2021.644109. eCollection 2021. Front Oncol. 2021. PMID: 33869035 Free PMC article. Review.
-
Extracellular Vesicles in Bone Metastasis: Key Players in the Tumor Microenvironment and Promising Therapeutic Targets.Int J Mol Sci. 2020 Sep 12;21(18):6680. doi: 10.3390/ijms21186680. Int J Mol Sci. 2020. PMID: 32932657 Free PMC article. Review.
-
Breast Cancer Derived Extracellular Vesicles in Bone Metastasis Induction and Their Clinical Implications as Biomarkers.Int J Mol Sci. 2020 May 18;21(10):3573. doi: 10.3390/ijms21103573. Int J Mol Sci. 2020. PMID: 32443642 Free PMC article. Review.
-
The Emerging Roles of Extracellular Vesicles in Osteosarcoma.Front Oncol. 2019 Dec 3;9:1342. doi: 10.3389/fonc.2019.01342. eCollection 2019. Front Oncol. 2019. PMID: 31850225 Free PMC article. Review.
Cited by
-
Effectiveness and Safety of the Traditional Chinese Medicine Treatment (HuoxueHuayu Therapy) for Malignant Tumors: A Systematic Review and Meta-Analysis.Evid Based Complement Alternat Med. 2022 Aug 25;2022:7944063. doi: 10.1155/2022/7944063. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 38094221 Free PMC article. Review.
-
The impacts of exosomes on bone metastatic progression and their potential clinical utility.Bone Rep. 2022 Jul 21;17:101606. doi: 10.1016/j.bonr.2022.101606. eCollection 2022 Dec. Bone Rep. 2022. PMID: 35910404 Free PMC article.
-
From Hypoxia to Bone: Reprogramming the Prostate Cancer Metastatic Cascade.Int J Mol Sci. 2025 Aug 1;26(15):7452. doi: 10.3390/ijms26157452. Int J Mol Sci. 2025. PMID: 40806577 Free PMC article. Review.
-
miR-92a-3p encapsulated in bone metastatic mammary tumor cell-derived extracellular vesicles modulates mature osteoclast longevity.Cancer Sci. 2022 Dec;113(12):4219-4229. doi: 10.1111/cas.15557. Epub 2022 Sep 27. Cancer Sci. 2022. PMID: 36053115 Free PMC article.
-
The basic characteristics of extracellular vesicles and their potential application in bone sarcomas.J Nanobiotechnology. 2021 Sep 17;19(1):277. doi: 10.1186/s12951-021-01028-7. J Nanobiotechnology. 2021. PMID: 34535153 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

