Molecular Components of Store-Operated Calcium Channels in the Regulation of Neural Stem Cell Physiology, Neurogenesis, and the Pathology of Huntington's Disease
- PMID: 33869222
- PMCID: PMC8047111
- DOI: 10.3389/fcell.2021.657337
Molecular Components of Store-Operated Calcium Channels in the Regulation of Neural Stem Cell Physiology, Neurogenesis, and the Pathology of Huntington's Disease
Abstract
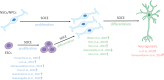
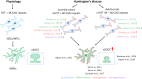
One of the major Ca2+ signaling pathways is store-operated Ca2+ entry (SOCE), which is responsible for Ca2+ flow into cells in response to the depletion of endoplasmic reticulum Ca2+ stores. SOCE and its molecular components, including stromal interaction molecule proteins, Orai Ca2+ channels, and transient receptor potential canonical channels, are involved in the physiology of neural stem cells and play a role in their proliferation, differentiation, and neurogenesis. This suggests that Ca2+ signaling is an important player in brain development. Huntington's disease (HD) is an incurable neurodegenerative disorder that is caused by polyglutamine expansion in the huntingtin (HTT) protein, characterized by the loss of γ-aminobutyric acid (GABA)-ergic medium spiny neurons (MSNs) in the striatum. However, recent research has shown that HD is also a neurodevelopmental disorder and Ca2+ signaling is dysregulated in HD. The relationship between HD pathology and elevations of SOCE was demonstrated in different cellular and mouse models of HD and in induced pluripotent stem cell-based GABAergic MSNs from juvenile- and adult-onset HD patient fibroblasts. The present review discusses the role of SOCE in the physiology of neural stem cells and its dysregulation in HD pathology. It has been shown that elevated expression of STIM2 underlying the excessive Ca2+ entry through store-operated calcium channels in induced pluripotent stem cell-based MSNs from juvenile-onset HD. In the light of the latest findings regarding the role of Ca2+ signaling in HD pathology we also summarize recent progress in the in vitro differentiation of MSNs that derive from different cell sources. We discuss advances in the application of established protocols to obtain MSNs from fetal neural stem cells/progenitor cells, embryonic stem cells, induced pluripotent stem cells, and induced neural stem cells and the application of transdifferentiation. We also present recent progress in establishing HD brain organoids and their potential use for examining HD pathology and its treatment. Moreover, the significance of stem cell therapy to restore normal neural cell function, including Ca2+ signaling in the central nervous system in HD patients will be considered. The transplantation of MSNs or their precursors remains a promising treatment strategy for HD.
Keywords: Ca2+ homeostasis; Huntington’s disease; brain organoids; induced pluripotent stem cells; neural stem cells; store-operated Ca2+ channels; store-operated Ca2+ entry.
Copyright © 2021 Latoszek and Czeredys.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Dysregulation of Neuronal Calcium Signaling via Store-Operated Channels in Huntington's Disease.Front Cell Dev Biol. 2020 Dec 23;8:611735. doi: 10.3389/fcell.2020.611735. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33425919 Free PMC article. Review.
-
STIM2 Mediates Excessive Store-Operated Calcium Entry in Patient-Specific iPSC-Derived Neurons Modeling a Juvenile Form of Huntington's Disease.Front Cell Dev Biol. 2021 Feb 2;9:625231. doi: 10.3389/fcell.2021.625231. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33604336 Free PMC article.
-
Huntingtin-Associated Protein 1A Regulates Store-Operated Calcium Entry in Medium Spiny Neurons From Transgenic YAC128 Mice, a Model of Huntington's Disease.Front Cell Neurosci. 2018 Oct 26;12:381. doi: 10.3389/fncel.2018.00381. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30455632 Free PMC article.
-
Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington's Disease Mouse Model.J Neurosci. 2016 Jan 6;36(1):125-41. doi: 10.1523/JNEUROSCI.1038-15.2016. J Neurosci. 2016. PMID: 26740655 Free PMC article.
-
On the Role of Store-Operated Calcium Entry in Acute and Chronic Neurodegenerative Diseases.Front Mol Neurosci. 2018 Mar 22;11:87. doi: 10.3389/fnmol.2018.00087. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29623030 Free PMC article. Review.
Cited by
-
Environmental adversity, endoplasmic reticulum stress, and neurogenesis.Neurotoxicology. 2025 Jul;109:32-45. doi: 10.1016/j.neuro.2025.05.010. Epub 2025 May 31. Neurotoxicology. 2025. PMID: 40456492 Review.
-
Patient-Specific iPSCs-Based Models of Neurodegenerative Diseases: Focus on Aberrant Calcium Signaling.Int J Mol Sci. 2022 Jan 6;23(2):624. doi: 10.3390/ijms23020624. Int J Mol Sci. 2022. PMID: 35054808 Free PMC article. Review.
-
Ethanol Causes Cell Death and Neuronal Differentiation Defect During Initial Neurogenesis of the Neural Retina by Disrupting Calcium Signaling in Human Retinal Organoids.Stem Cell Rev Rep. 2023 Nov;19(8):2790-2806. doi: 10.1007/s12015-023-10604-3. Epub 2023 Aug 21. Stem Cell Rev Rep. 2023. PMID: 37603136
-
Connection Lost, MAM: Errors in ER-Mitochondria Connections in Neurodegenerative Diseases.Brain Sci. 2021 Oct 28;11(11):1437. doi: 10.3390/brainsci11111437. Brain Sci. 2021. PMID: 34827436 Free PMC article. Review.
-
Therapeutic role of neural stem cells in neurological diseases.Front Bioeng Biotechnol. 2024 Mar 7;12:1329712. doi: 10.3389/fbioe.2024.1329712. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38515621 Free PMC article. Review.
References
-
- Al-Gharaibeh A., Culver R., Stewart A. N., Srinageshwar B., Spelde K., Frollo L., et al. (2017). Induced Pluripotent Stem Cell-Derived Neural Stem Cell Transplantations Reduced Behavioral Deficits and Ameliorated Neuropathological Changes in YAC128 Mouse Model of Huntington’s Disease. Front Neurosci 11:628. 10.3389/fnins.2017.00628 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

