A Nanomule Peptide Carrier Delivers siRNA Across the Intact Blood-Brain Barrier to Attenuate Ischemic Stroke
- PMID: 33869275
- PMCID: PMC8044710
- DOI: 10.3389/fmolb.2021.611367
A Nanomule Peptide Carrier Delivers siRNA Across the Intact Blood-Brain Barrier to Attenuate Ischemic Stroke
Erratum in
-
Corrigendum: A Nanomule Peptide Carrier Delivers siRNA Across the Intact Blood-Brain Barrier to Attenuate Ischemic Stroke.Front Mol Biosci. 2021 May 7;8:687587. doi: 10.3389/fmolb.2021.687587. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026851 Free PMC article.
Abstract
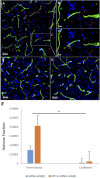
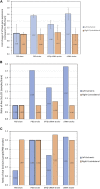
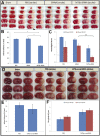
The blood-brain barrier (BBB) hinders the distribution of therapeutics intended for treatment of neuroinflammation (NI) of the central nervous system. A twelve-amino acid peptide that transcytoses the BBB, termed MTfp, was chemically conjugated to siRNA to create a novel peptide-oligonucleotide conjugate (POC), directed to downregulate NOX4, a gene thought responsible for oxidative stress in ischemic stroke. The MTfp-NOX4 POC has the ability to cross the intact BBB and knockdown NOX4 expression in the brain. Following induction of ischemic stroke, animals pretreated with the POC exhibited significantly smaller infarcts; accompanied by increased protection against neurological deterioration and improved recovery. The data demonstrates that the MTfp can act as a nanomule to facilitate BBB transcytosis of siRNAs; where the NOX-4 specific siRNA moiety can elicit effective therapeutic knockdown of a gene responsible for oxidative stress in the central nervous system. This study is the first to conclusively demonstrate both siRNA-carrier delivery and therapeutic efficacy in any CNS disease model where the BBB remains intact and thus offers new avenues for potential treatments of oxidative stress underlying neuroinflammation in a variety of neuropathologies that are currently refractory to existing therapies.
Keywords: MTfp; NOX4; blood-brain barrier; peptide-oligonucleotide conjugate; siRNA; stroke.
Copyright © 2021 Eyford, Singh, Abraham, Munro, Choi, Hill, Hildebrandt, Welch, Vitalis, Gabathuler, Gordon, Adomat, Guns, Lu, Pfeifer, Tian and Jefferies.
Conflict of interest statement
TV, RG, and MMT received funding from Bioasis Technologies Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures

Similar articles
-
Oxidative Injury in Ischemic Stroke: A Focus on NADPH Oxidase 4.Oxid Med Cell Longev. 2022 Feb 3;2022:1148874. doi: 10.1155/2022/1148874. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35154560 Free PMC article. Review.
-
Corrigendum: A Nanomule Peptide Carrier Delivers siRNA Across the Intact Blood-Brain Barrier to Attenuate Ischemic Stroke.Front Mol Biosci. 2021 May 7;8:687587. doi: 10.3389/fmolb.2021.687587. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026851 Free PMC article.
-
Discovery of a Highly Conserved Peptide in the Iron Transporter Melanotransferrin that Traverses an Intact Blood Brain Barrier and Localizes in Neural Cells.Front Neurosci. 2021 Jun 2;15:596976. doi: 10.3389/fnins.2021.596976. eCollection 2021. Front Neurosci. 2021. PMID: 34149342 Free PMC article.
-
Glycocalyx is critical for blood-brain barrier integrity by suppressing caveolin1-dependent endothelial transcytosis following ischemic stroke.Brain Pathol. 2022 Jan;32(1):e13006. doi: 10.1111/bpa.13006. Epub 2021 Jul 19. Brain Pathol. 2022. PMID: 34286899 Free PMC article.
-
Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities.Stroke. 2022 May;53(5):1473-1486. doi: 10.1161/STROKEAHA.122.036946. Epub 2022 Apr 7. Stroke. 2022. PMID: 35387495 Free PMC article. Review.
Cited by
-
RNA-based therapeutics for neurological diseases.RNA Biol. 2022;19(1):176-190. doi: 10.1080/15476286.2021.2021650. Epub 2021 Dec 31. RNA Biol. 2022. PMID: 35067193 Free PMC article. Review.
-
Nucleic acid-based therapeutics for the treatment of central nervous system disorders.Front Genet. 2023 Aug 16;14:1250276. doi: 10.3389/fgene.2023.1250276. eCollection 2023. Front Genet. 2023. PMID: 37662844 Free PMC article. Review.
-
Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates.Nat Biotechnol. 2022 Oct;40(10):1500-1508. doi: 10.1038/s41587-022-01334-x. Epub 2022 Jun 2. Nat Biotechnol. 2022. PMID: 35654979
-
Directing the Way-Receptor and Chemical Targeting Strategies for Nucleic Acid Delivery.Pharm Res. 2023 Jan;40(1):47-76. doi: 10.1007/s11095-022-03385-w. Epub 2022 Sep 15. Pharm Res. 2023. PMID: 36109461 Free PMC article. Review.
-
Oxidative Injury in Ischemic Stroke: A Focus on NADPH Oxidase 4.Oxid Med Cell Longev. 2022 Feb 3;2022:1148874. doi: 10.1155/2022/1148874. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35154560 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

