Perspectives on RNA Vaccine Candidates for COVID-19
- PMID: 33869282
- PMCID: PMC8044912
- DOI: 10.3389/fmolb.2021.635245
Perspectives on RNA Vaccine Candidates for COVID-19
Abstract
With the current outbreak caused by SARS-CoV-2, vaccination is acclaimed as a public health care priority. Rapid genetic sequencing of SARS-CoV-2 has triggered the scientific community to search for effective vaccines. Collaborative approaches from research institutes and biotech companies have acknowledged the use of viral proteins as potential vaccine candidates against COVID-19. Nucleic acid (DNA or RNA) vaccines are considered the next generation vaccines as they can be rapidly designed to encode any desirable viral sequence including the highly conserved antigen sequences. RNA vaccines being less prone to host genome integration (cons of DNA vaccines) and anti-vector immunity (a compromising factor of viral vectors) offer great potential as front-runners for universal COVID-19 vaccine. The proof of concept for RNA-based vaccines has already been proven in humans, and the prospects for commercialization are very encouraging as well. With the emergence of COVID-19, mRNA-1273, an mRNA vaccine developed by Moderna, Inc. was the first to enter human trials, with the first volunteer receiving the dose within 10 weeks after SARS-CoV-2 genetic sequencing. The recent interest in mRNA vaccines has been fueled by the state of the art technologies that enhance mRNA stability and improve vaccine delivery. Interestingly, as per the "Draft landscape of COVID-19 candidate vaccines" published by the World Health Organization (WHO) on December 29, 2020, seven potential RNA based COVID-19 vaccines are in different stages of clinical trials; of them, two candidates already received emergency use authorization, and another 22 potential candidates are undergoing pre-clinical investigations. This review will shed light on the rationality of RNA as a platform for vaccine development against COVID-19, highlighting the possible pros and cons, lessons learned from the past, and the future prospects.
Keywords: COVID-19; SARS-CoV-2; conventional RNA; mRNA; mRNA-1273; replicons; self-amplifying RNA; vaccine.
Copyright © 2021 Borah, Deb, Al-Shar’i, Dahabiyeh, Venugopala, Singh, Shinu, Hussain, Deka, Chandrasekaran and Jaradat.
Conflict of interest statement
The authors declare that the review article was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
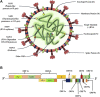

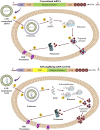
References
-
- Alberer M., Gnad-Vogt U., Hong H. S., Mehr K. T., Backert L., Finak G., et al. (2017). Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 390, 1511–1520. 10.1016/S0140-6736(17)31665-3 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

