Improvement of HSV-1 based amplicon vectors for a safe and long-lasting gene therapy in non-replicating cells
- PMID: 33869657
- PMCID: PMC8044385
- DOI: 10.1016/j.omtm.2021.03.020
Improvement of HSV-1 based amplicon vectors for a safe and long-lasting gene therapy in non-replicating cells
Abstract
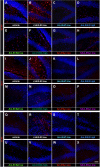
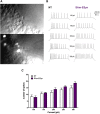
A key factor for developing gene therapy strategies for neurological disorders is the availability of suitable vectors. Currently, the most advanced are adeno-associated vectors that, while being safe and ensuring long-lasting transgene expression, have a very limited cargo capacity. In contrast, herpes simplex virus-based amplicon vectors can host huge amounts of foreign DNA, but concerns exist about their safety and ability to express transgenes long-term. We aimed at modulating and prolonging amplicon-induced transgene expression kinetics in vivo using different promoters and preventing transgene silencing. To pursue the latter, we deleted bacterial DNA sequences derived from vector construction and shielded the transgene cassette using AT-rich and insulator-like sequences (SAm technology). We employed luciferase and GFP as reporter genes. To determine transgene expression kinetics, we injected vectors in the hippocampus of mice that were longitudinally scanned for bioluminescence for 6 months. To evaluate safety, we analyzed multiple markers of damage and performed patch clamp electrophysiology experiments. All vectors proved safe, and we managed to modulate the duration of transgene expression, up to obtaining a stable, long-lasting expression using the SAm technology. Therefore, these amplicon vectors represent a flexible, efficient, and safe tool for gene delivery in the brain.
Keywords: Central Nervous System; amplicon vector; herpes simplex virus; hippocampus.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Simonato M., Manservigi R., Marconi P., Glorioso J. Gene transfer into neurones for the molecular analysis of behaviour: focus on herpes simplex vectors. Trends Neurosci. 2000;23:183–190. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

