Six Sigma performance of quality indicators in total testing process of point-of-care glucose measurement: A two-year review
- PMID: 33869708
- PMCID: PMC8042413
- DOI: 10.1016/j.plabm.2021.e00215
Six Sigma performance of quality indicators in total testing process of point-of-care glucose measurement: A two-year review
Abstract
Objectives: The error rate in the total testing process (TTP) of point-of-care (POC) glucose measurement remains high although a total quality management system has been applied. Quality indicators (QIs) in the TTP of glucose meter were established via risk assessment. Their two-year Six Sigma values were reviewed for quality improvement.
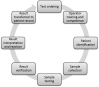
Design: The TTP of POC glucose measurement was mapped to identify risks in key steps. The risks were assessed for their frequency and severity of impact on patient safety. Whenever possible, measurable data from the data management system and other sources was collected to establish QIs for risk monitoring. Average Six Sigma value of each QI in the last two years was calculated for acceptance and for determining corrective action.
Results: 29 risks were identified in eight key steps of the TTP. Eight QIs were established for monitoring six risks and three QIs for two accepted risks were established for improving operator testing skill. The QIs had a good coverage to key steps. Two, five and four QIs showed Six Sigma values <3, 3-4 and >4 respectively. Six Sigma values of two QIs related to quality control (QC) testing were improved by using meters with accurate QC sample loading.
Conclusions: The establishment of QIs for glucose measurement by risk assessment with measurable data from the data management system and on Six sigma scale was effective, efficient, and manageable. Most of QIs' Six Sigma values were between 3 and 5, which could be improved by using upgraded meters.
Keywords: Glucose meter; Point-of-care testing; Quality indicator; Risk assessment; Six sigma.
© 2021 The Authors.
Conflict of interest statement
There are no known conflicts of interest associated with this publication.
Figures
References
-
- Plebani M. Does POCT reduce the risk of error in laboratory testing? Clin. Chim. Acta. 2009;404:59–64. - PubMed
-
- O’Kane M.J., McManus P., McGowan N., Lynch P.L. Quality error rates in point-of-care testing. Clin. Chem. 2011;57:1267–1271. - PubMed
-
- International Organization for Standardization, ISO 14971:2019 Medical Devices - Application of Risk Management to Medical Devices.
LinkOut - more resources
Full Text Sources
Other Literature Sources