SQ3370 Activates Cytotoxic Drug via Click Chemistry at Tumor and Elicits Sustained Responses in Injected & Non-injected Lesions
- PMID: 33869738
- PMCID: PMC8049173
- DOI: 10.1002/adtp.202000243
SQ3370 Activates Cytotoxic Drug via Click Chemistry at Tumor and Elicits Sustained Responses in Injected & Non-injected Lesions
Abstract
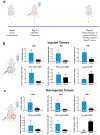
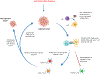
While systemic immuno-oncology therapies have shown remarkable success, only a limited subset of patients benefit from them. Our Click Activated Protodrugs Against Cancer (CAPAC™) Platform is a click chemistry-based approach that activates cancer drugs at a specific tumor with minimal systemic toxicity. CAPAC Platform is agnostic to tumor characteristics that can vary across patients and hence applicable to several types of tumors. We describe the benefits of SQ3370 (lead candidate of CAPAC) to achieve systemic anti-tumor responses in mice bearing two tumors. SQ3370 consists of a biopolymer, injected in a single lesion, followed by systemic doses of an attenuated protodrug™ of doxorubicin (Dox). SQ3370 was well-tolerated at 5.9-times the maximum dose of conventional Dox, increased survival by 63% and induced a systemic anti-tumor response against injected and non-injected lesions. The sustained anti-tumor response also correlated with immune activation measured at both lesions. SQ3370 could potentially benefit patients with micro-metastatic lesions.
Keywords: Bioorthogonal Chemistry; abscopal effect; anenestic response; immuno-oncology; local drug activation; platform technology.
Conflict of interest statement
Conflict of Interest S. Srinivasan, N. A. Yee, M. Zakharian, A. Mahmoodi, J. M. Mejia Oneto are paid employees and shareholders of Shasqi, Inc. (Shasqi). J.M. Mejia Oneto is the Founder and CEO of Shasqi. M. Royzen is a shareholder of Shasqi.
Figures



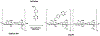
References
-
- Tian F; Lu Y; Manibusan A; Sellers A; Tran H; Sun Y; Phuong T; Barnett R; Hehli B; Song F; DeGuzman MJ; Ensari S; Pinkstaff JK; Sullivan LM; Biroc SL; Cho H; Schultz PG; di Joseph J; Dougher M; Ma D; Dushin R; Leal M; Tchistiakova L; Feyfant E; Gerber HP; Sapra P A General Approach to Site-Specific Antibody Drug Conjugates. Proceedings of the National Academy of Sciences of the United States of America 2014, 111 (5), 1766–1771. 10.1073/pnas.1321237111. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
