How Many CO2 Bubbles in a Glass of Beer?
- PMID: 33869947
- PMCID: PMC8047704
- DOI: 10.1021/acsomega.1c00256
How Many CO2 Bubbles in a Glass of Beer?
Abstract
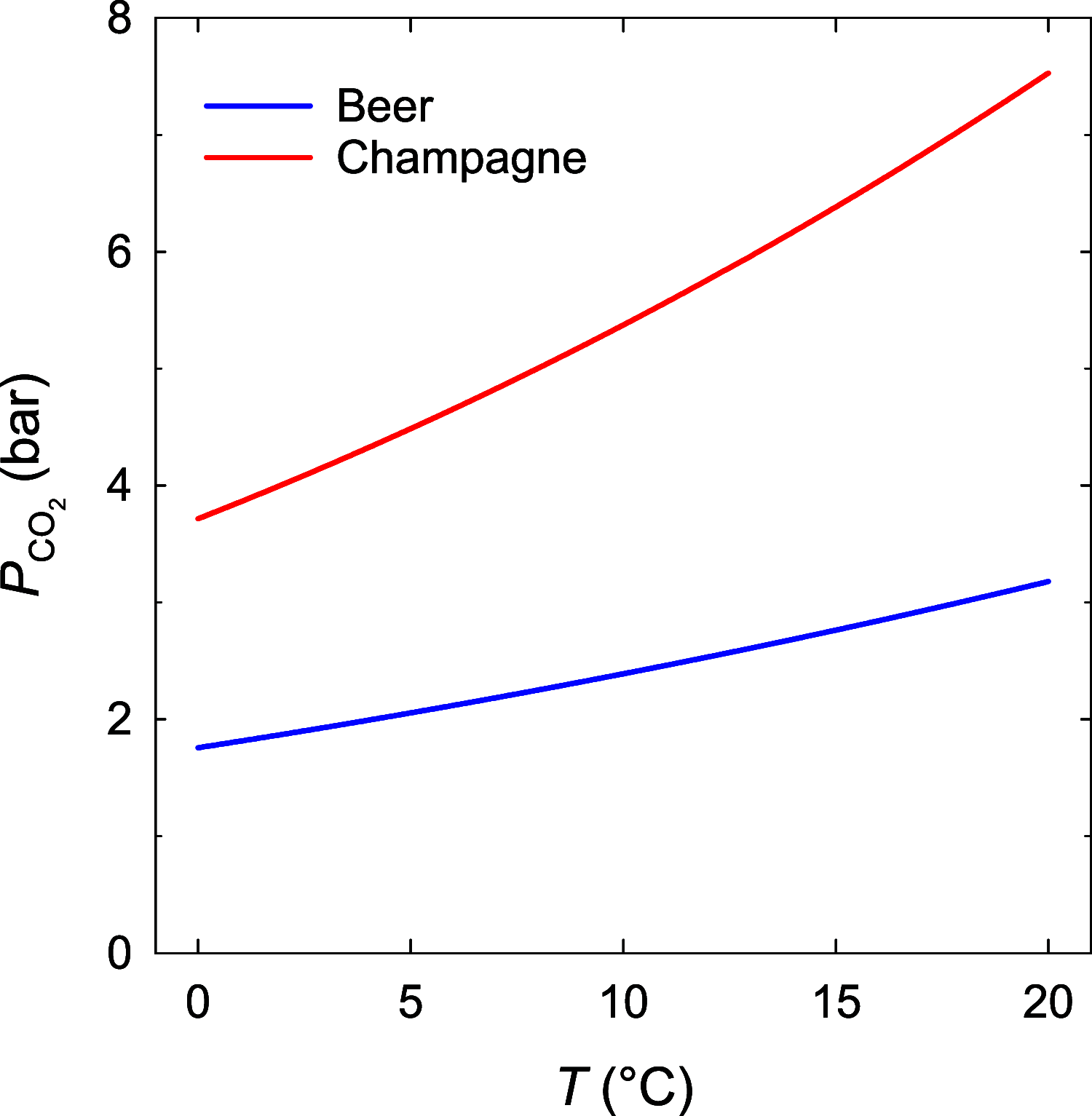
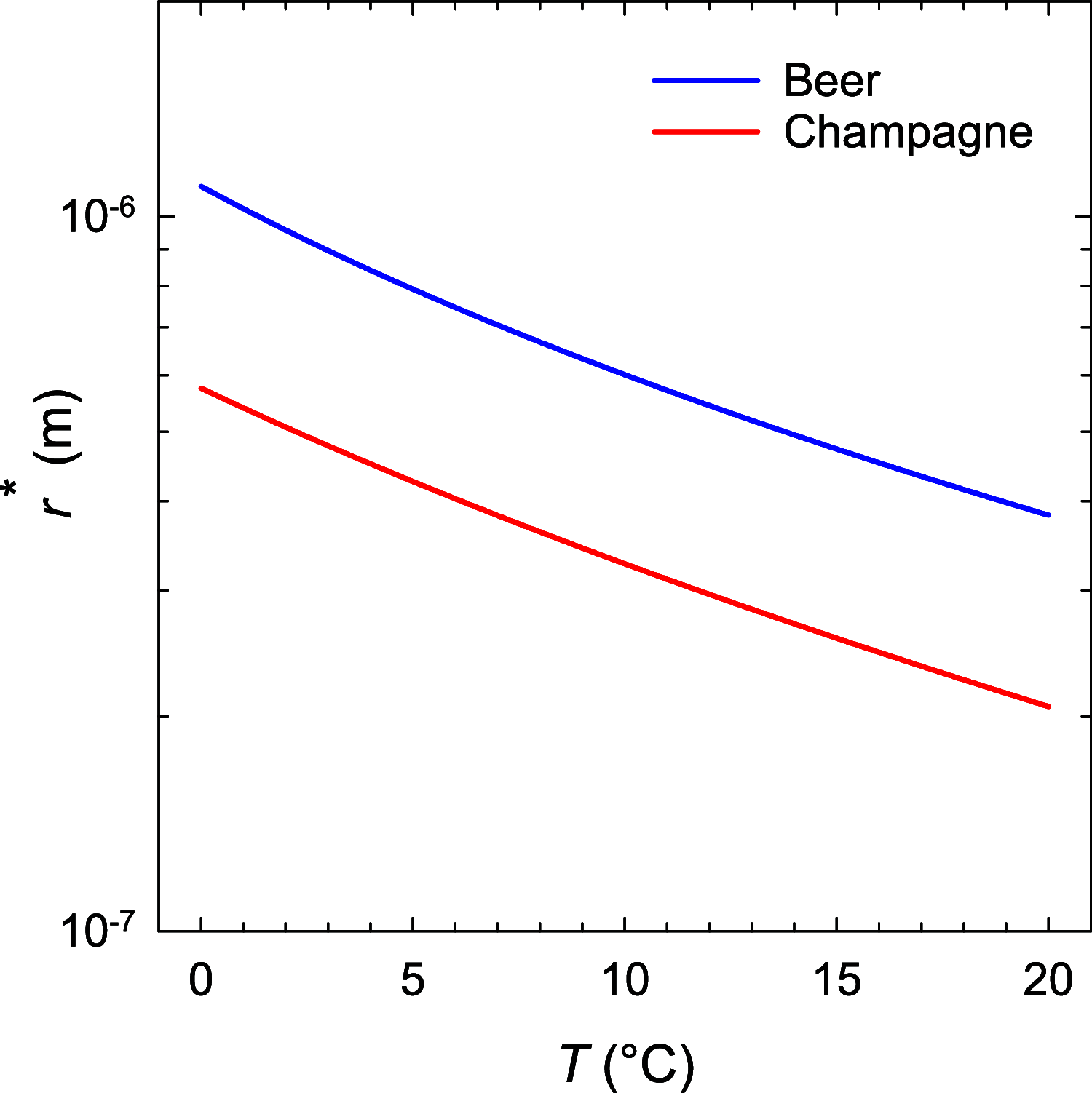
The number of bubbles likely to form in a glass of beer is the result of the fine interplay between dissolved CO2, tiny particles or glass imperfections acting as bubble nucleation sites, and ascending bubble dynamics. Experimental and theoretical developments about the thermodynamic equilibrium of dissolved and gas-phase carbon dioxide (CO2) were made relevant to the bottling and service of a commercial lager beer, with 5% alcohol by volume and a concentration of dissolved CO2 close to 5.5 g L-1. The critical radius and the subsequent critical concentration of dissolved CO2 needed to trigger heterogeneous nucleation of CO2 bubbles from microcrevices once the beer was dispensed in a glass were derived. The subsequent total number of CO2 bubbles likely to form in a single glass of beer was theoretically approached as a function of the various key parameters under standard tasting conditions. The present results with the lager beer were compared with previous sets of data measured with a standard commercial Champagne wine (with 12.5% alcohol by volume and a concentration of dissolved CO2 close to 11 g L-1).
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
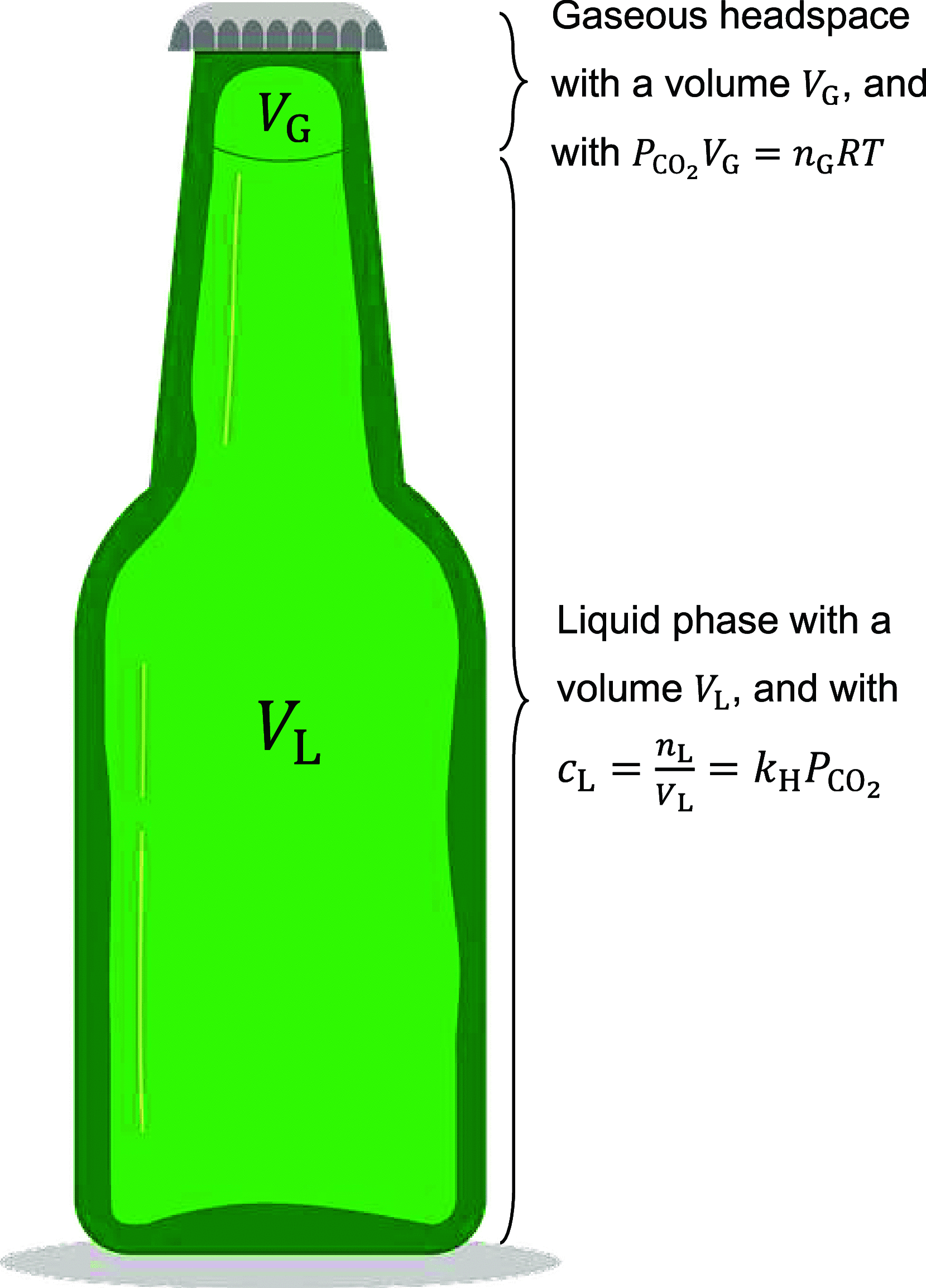




References
-
- Liu L.; Wang J.; Rosenberg D.; Zhao H.; Lengyel G.; Nadel D. Fermented beverage and food storage in 13 000 y-old stone mortars at Raqefet Cave, Israel: Investigating Natufian ritual feasting. J. Archaeol. Sci. 2018, 21, 783–793. 10.1016/j.jasrep.2018.08.008. - DOI
-
- McGovern P.Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages; University of California Press: Berkeley, 2011.
-
- Beer production worldwide from 1998 to 2019. https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed Dec 25, 2020).
-
- Beer Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021-2026. https://www.imarcgroup.com/beer-market (accessed Dec 25, 2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources

