Synthesis and Biological Evaluation of Novel Benzhydrylpiperazine-Coupled Nitrobenzenesulfonamide Hybrids
- PMID: 33869953
- PMCID: PMC8047747
- DOI: 10.1021/acsomega.1c00369
Synthesis and Biological Evaluation of Novel Benzhydrylpiperazine-Coupled Nitrobenzenesulfonamide Hybrids
Abstract
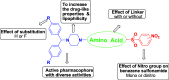
A series of novel benzhydryl piperazine-coupled nitrobenzenesulfonamide hybrids were synthesized with good to excellent yields. They were tested for in vitro inhibition of mycobacterial activity against the Mycobacterium tuberculosis H37Rv strain, in vitro cytotoxicity MTT (RAW 264.7cells) assay, nutrient starvation (H37Rv strain), and ability to block Cav3.2 T-type calcium channels. Novel hybrids did not inhibit T-type calcium channels, whereas they showed excellent antituberculosis (TB) activity and low cytotoxicity with a selectivity index of >30. A direct impact of the amino acid linker was not observed. Studied hybrids exhibited good inhibition activities, and the 2,4-dinitrobenzenesulfonamide group emerged as a promising scaffold for further drug design by hybridization approaches for anti-TB therapy.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- https://www.tballiance.org/why-new-tb-drugs/global-pandemic (accessed March 2, 2021).
-
- Abubakar I.; Zignol M.; Falzon D.; Raviglione M.; Ditiu L.; Masham S.; Adetifa I.; Ford N.; Cox H.; Lawn S. D.; Marais B. J.; McHugh T. D.; Mwaba P.; Bates M.; Lipman M.; Zijenah L.; Logan S.; McNerney R.; Zumla A.; Sarda K.; Nahid P.; Hoelscher M.; Pletschette M.; Memish Z. A.; Kim P.; Hafner R.; Cole S.; Migliori G. B.; Maeurer M.; Schito M.; Zumla A. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect. Dis. 2013, 13, 529–539. 10.1016/s1473-3099(13)70030-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

