Ligand Binding Prolongs Androgen Receptor Protein Half-Life by Reducing its Degradation
- PMID: 33869982
- PMCID: PMC8043068
- DOI: 10.1210/jendso/bvab035
Ligand Binding Prolongs Androgen Receptor Protein Half-Life by Reducing its Degradation
Abstract
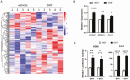
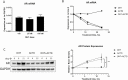
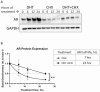
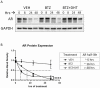
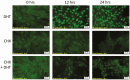
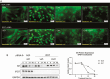
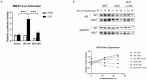
Androgens are important in female reproduction, but the molecular actions of androgens in female reproductive tissues are not fully understood. We investigated the androgen-responsive transcriptome in human and mouse granulosa cells (GCs) and surprisingly found that the gene-regulation activity of androgen receptor (AR) in these cells is negligible. We then investigated extranuclear actions of AR and found that in human and mouse GCs, as well as in prostate cancer cells, dihydrotestosterone (DHT) dramatically increases the half-life of its own receptor protein. Using the human granulosa-like KGN cells, we show that this effect is not the result of increased AR gene transcription or protein synthesis, nor is it fully abrogated by proteasome inhibition. Knockdown of PTEN, which contributes to degradation of cytoplasmic AR, did not diminish AR accumulation in the presence of DHT. Using immunofluorescence cellular localization studies, we show that nuclear AR is selectively protected from degradation in the presence of DHT. Knockdown of importin 7 expression, a potential regulator of AR nuclear import, does not affect DHT-mediated nuclear accumulation of AR, suggesting importin 7-independent nuclear import of AR in GCs. Further, DNA binding is not required for this protective mechanism. In summary, we show that ligand binding sequesters AR in the nucleus through enhanced nuclear localization independent of DNA binding, thereby protecting it from proteasome degradation in the cytoplasm. This phenomenon distinguishes AR from other sex steroid receptors and may have physiological significance through a positive feedback loop in which androgen induces its own activity in male and female reproductive tissues.
Keywords: androgen; granulosa; ovary; prostate; proteasome; transcriptome.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Lyon MF, Glenister PH. Reduced reproductive performance in androgen-resistant Tfm/Tfm female mice. Proc R Soc Lond B Biol Sci. 1980;208(1170):1-12. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
