Phylobioactive hotspots in plant resources used to treat Chagas disease
- PMID: 33870129
- PMCID: PMC8040286
- DOI: 10.1016/j.isci.2021.102310
Phylobioactive hotspots in plant resources used to treat Chagas disease
Abstract
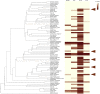
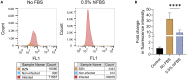
Globally, more than six million people are infected with Trypanosoma cruzi, the causative protozoan parasite of the vector-borne Chagas disease (CD). We conducted a cross-sectional ethnopharmacological field study in Bolivia among different ethnic groups where CD is hyperendemic. A total of 775 extracts of botanical drugs used in Bolivia in the context of CD and botanical drugs from unrelated indications from the Mediterranean De Materia Medica compiled by Dioscorides two thousand years ago were profiled in a multidimensional assay uncovering different antichagasic natural product classes. Intriguingly, the phylobioactive anthraquinone hotspot matched the antichagasic activity of Senna chloroclada, the taxon with the strongest ethnomedical consensus for treating CD among the Izoceño-Guaraní. Testing common 9,10-anthracenedione derivatives in T. cruzi cellular infection assays demarcates hydroxyanthraquinone as a potential antichagasic lead scaffold. Our study systematically uncovers in vitro antichagasic phylogenetic hotspots in the plant kingdom as a potential resource for drug discovery based on ethnopharmacological hypotheses.
Keywords: Bioactive Plant Product; Biological Sciences; Ethnobotany; Ethnopharmacology; Natural Product Chemistry; Plant Biology; Plants.
© 2021 The Authors.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





Similar articles
-
Cultural perception of triatomine bugs and Chagas disease in Bolivia: a cross-sectional field study.Parasit Vectors. 2019 Jun 10;12(1):291. doi: 10.1186/s13071-019-3546-0. Parasit Vectors. 2019. PMID: 31182163 Free PMC article.
-
Pharmacopoeia in a shamanistic society: the Izoceño-Guaraní (Bolivian Chaco).J Ethnopharmacol. 2004 Apr;91(2-3):189-208. doi: 10.1016/j.jep.2003.09.013. J Ethnopharmacol. 2004. PMID: 15120439
-
Back to the roots: A quantitative survey of herbal drugs in Dioscorides' De Materia Medica (ex Matthioli, 1568).Phytomedicine. 2016 Sep 15;23(10):1043-52. doi: 10.1016/j.phymed.2016.06.016. Epub 2016 Jun 23. Phytomedicine. 2016. PMID: 27444350 Review.
-
Bioactivity-guided isolation of trypanocidal coumarins and dihydro-pyranochromones from selected Apiaceae plant species.Phytochemistry. 2023 Sep;213:113770. doi: 10.1016/j.phytochem.2023.113770. Epub 2023 Jun 16. Phytochemistry. 2023. PMID: 37331573
-
Chagas Disease and Coumarins: A Review of Natural and Synthetic Coumarins As Anti-Trypanosoma Cruzi Agents.Mini Rev Med Chem. 2021;21(13):1701-1717. doi: 10.2174/1389557521666201229125931. Mini Rev Med Chem. 2021. PMID: 33372872 Review.
Cited by
-
Harnessing Phytonanotechnology to Tackle Neglected Parasitic Diseases: Focus on Chagas Disease and Malaria.Pharmaceutics. 2025 Aug 12;17(8):1043. doi: 10.3390/pharmaceutics17081043. Pharmaceutics. 2025. PMID: 40871066 Free PMC article. Review.
-
Semisynthetic Ecdysteroid Cinnamate Esters and tert-Butyl Oxime Ether Derivatives with Trypanocidal Activity.J Nat Prod. 2024 Oct 25;87(10):2478-2486. doi: 10.1021/acs.jnatprod.4c00811. Epub 2024 Oct 17. J Nat Prod. 2024. PMID: 39417525 Free PMC article.
-
Anti-trypanosomal screening of Salvadoran flora.J Nat Med. 2022 Jan;76(1):259-267. doi: 10.1007/s11418-021-01562-6. Epub 2021 Sep 16. J Nat Med. 2022. PMID: 34529189 Free PMC article.
-
Are we romanticizing traditional knowledge? A plea for more experimental studies in ethnobiology.J Ethnobiol Ethnomed. 2024 May 26;20(1):56. doi: 10.1186/s13002-024-00697-6. J Ethnobiol Ethnomed. 2024. PMID: 38797828 Free PMC article.
-
In Vitro Anti-Trypanosoma cruzi Activity of Halophytes from Southern Portugal Reloaded: A Special Focus on Sea Fennel (Crithmum maritimum L.).Plants (Basel). 2021 Oct 20;10(11):2235. doi: 10.3390/plants10112235. Plants (Basel). 2021. PMID: 34834598 Free PMC article.
References
-
- Alonso J.R. El lapacho. Revista de Fitoterapia. 2000;1:107–117.
-
- de Araújo-Jorge T.C., Medrano-Mercado N. Chagas disease in Bolivia: a brief review of the urban phenomena. Revista Biomédica. 2009;20:236–244. http://www.revbiomed.uady.mx/pdf/rb092038.pdf
-
- de Arias A., Inchausti A., Ascurrat M., Fleitas N., Rodriguez E., Fournet A. In vitro activity and mutagenicity of bisbenzylisoquinolines and quinones againstTrypanosoma cruzi trypomastigotes. Phytother. Res. 1994;8:141–144.
-
- Barrett M., Burchmore R., Stich A., Lazzari J., Frasch A., Cazzulo J., Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

