RBD-specific polyclonal F(ab´)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial
- PMID: 33870149
- PMCID: PMC8037439
- DOI: 10.1016/j.eclinm.2021.100843
RBD-specific polyclonal F(ab´)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial
Abstract
Background: passive immunotherapy is a therapeutic alternative for patients with COVID-19. Equine polyclonal antibodies (EpAbs) could represent a source of scalable neutralizing antibodies against SARS-CoV-2.
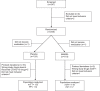
Methods: we conducted a double-blind, randomized, placebo-controlled trial to assess efficacy and safety of EpAbs (INM005) in hospitalized adult patients with moderate and severe COVID-19 pneumonia in 19 hospitals of Argentina. Primary endpoint was improvement in at least two categories in WHO ordinal clinical scale at day 28 or hospital discharge (ClinicalTrials.gov number NCT04494984).
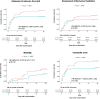
Findings: between August 1st and October 26th, 2020, a total of 245 patients were enrolled. Enrolled patients were assigned to receive two blinded doses of INM005 (n = 118) or placebo (n = 123). Median age was 54 years old, 65•1% were male and 61% had moderate disease at baseline. Median time from symptoms onset to study treatment was 6 days (interquartile range 5 to 8). No statistically significant difference was noted between study groups on primary endpoint (risk difference [95% IC]: 5•28% [-3•95; 14•50]; p = 0•15). Rate of improvement in at least two categories was statistically significantly higher for INM005 at days 14 and 21 of follow-up. Time to improvement in two ordinal categories or hospital discharge was 14•2 (± 0•7) days in the INM005 group and 16•3 (± 0•7) days in the placebo group, hazard ratio 1•31 (95% CI 1•0 to 1•74). Subgroup analyses showed a beneficial effect of INM005 over severe patients and in those with negative baseline antibodies. Overall mortality was 6•9% the INM005 group and 11•4% in the placebo group (risk difference [95% IC]: 0•57 [0•24 to 1•37]). Adverse events of special interest were mild or moderate; no anaphylaxis was reported.
Interpretation: Albeit not having reached the primary endpoint, we found clinical improvement of hospitalized patients with SARS-CoV-2 pneumonia, particularly those with severe disease.
© 2021 The Author(s).
Conflict of interest statement
MC, SS, VZ, LM, LS, FG received grants from Ministries of Science and Production of Argentina. MD, JF, GV, AB, FC, MFA, LB, RT, SL, DS, MI, VS, RS, PC, MMC, LA, HLL, AC, DC declare reimbursement for conduction of clinical trial as investigator of the study. PC, OS, YK report other funds from Inmunova S.A. EN, GL, WHB, SPLL report personal fees from Inmunova S.A. SM reports non-financial support from Inmunova S.A. AP, B de M, Gabriel L declare no competing interests. SPLL declare personal fees from Movement Disorders Society, Laboratorio Elea and Merck pharmaceuticals. MC, SS, VZ, LM, LS are employed by Inmunova S.A.
Figures

References
-
- https://www.worldometers.info/coronavirus/. COVID-19 coronavirus pandemic. (accessed January 15th, 2021).
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

