First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors
- PMID: 33870151
- PMCID: PMC8040281
- DOI: 10.1016/j.eclinm.2021.100797
First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors
Abstract
Background: We conducted a first-in-human dose-escalation study with the oral FASN inhibitor TVB-2640 to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D), as monotherapy and with a taxane.
Methods: This completed open-label outpatient study was conducted at 11 sites in the United States and United Kingdom. Patients with previously-treated advanced metastatic solid tumors and adequate performance status and organ function were eligible. TVB-2640 was administered orally daily until PD. Dose escalation initially followed an accelerated titration design that switched to a standard 3 + 3 design after Grade 2 toxicity occurred. Disease-specific cohorts were enrolled at the MTD. Statistical analyses were primarily descriptive. Safety analyses were performed on patients who received at least 1 dose of study drug. (Clinicaltrials.gov identifier NCT02223247).
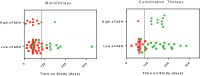
Findings: The study was conducted from 21 November 2013 to 07 February 2017. Overall, 136 patients received TVB-2640, 76 as monotherapy (weight-based doses of 60 mg/m2 to 240 mg/m2 and flat doses of 200 and 250 mg) and 60 in combination, (weight-based doses of 60 mg/m2 to 100 mg/m2 and flat dose of 200 mg) (55 paclitaxel, 5 docetaxel). DLTs with TVB-2640 were reversible skin and ocular effects. The MTD/RP2D was 100 mg/m2. The most common TEAEs (n,%) with TVB-2640 monotherapy were alopecia (46; 61%), PPE syndrome (35; 46%), fatigue (28; 37%), decreased appetite (20; 26%), and dry skin (17; 22%), and with TVB-2640+paclitaxel were fatigue (29 ; 53%), alopecia (25; 46%), PPE syndrome (25; 46%), nausea (22; 40%), and peripheral neuropathy (20; 36%). One fatal case of drug-related pneumonitis occurred with TVB-2640+paclitaxel; no other treatment-related deaths occurred. Target engagement (FASN inhibition) and inhibition of lipogenesis were demonstrated with TVB-2640. The disease control rate (DCR) with TVB-2640 monotherapy was 42%; no patient treated with monotherapy had a complete or partial response (CR or PR). In combination with paclitaxel, the PR rate was 11% and the DCR was 70%. Responses were seen across multiple tumor types, including in patients with KRASMUT NSCLC, ovarian, and breast cancer.
Interpretation: TVB-2640 demonstrated potent FASN inhibition and a predictable and manageable safety profile, primarily characterized by non-serious, reversible adverse events affecting skin and eyes. Further investigation of TVB-2640 in patients with solid tumors, particularly in KRASMUT lung, ovarian, and breast cancer, is warranted.
Funding: This trial was funded by 3-V Biosciences, Inc. (now known as Sagimet Biosciences Inc.).
Keywords: Biomarkers; Breast cancer; Clinical trials; Drug mechanisms; Gynecological cancers; Lung cancer; Pharmacology; Small molecule agents.
© 2021 The Authors.
Figures





References
-
- Al-Bahlani S., Al-Lawati H., Al-Adawi M. Fatty acid synthase regulates the chemosensitivity of breast cancer cells to cisplatin-induced apoptosis. Apoptosis. 2017;22(6):86576. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

