Genetic or pharmacological reduction of cholangiocyte senescence improves inflammation and fibrosis in the Mdr2 -/- mouse
- PMID: 33870156
- PMCID: PMC8044431
- DOI: 10.1016/j.jhepr.2021.100250
Genetic or pharmacological reduction of cholangiocyte senescence improves inflammation and fibrosis in the Mdr2 -/- mouse
Abstract
Background & aims: Cholangiocyte senescence is important in the pathogenesis of primary sclerosing cholangitis (PSC). We found that CDKN2A (p16), a cyclin-dependent kinase inhibitor and mediator of senescence, was increased in cholangiocytes of patients with PSC and from a PSC mouse model (multidrug resistance 2; Mdr2 -/-). Given that recent data suggest that a reduction of senescent cells is beneficial in different diseases, we hypothesised that inhibition of cholangiocyte senescence would ameliorate disease in Mdr2 -/- mice.
Methods: We used 2 novel genetic murine models to reduce cholangiocyte senescence: (i) p16Ink4a apoptosis through targeted activation of caspase (INK-ATTAC)xMdr2 -/-, in which the dimerizing molecule AP20187 promotes selective apoptotic removal of p16-expressing cells; and (ii) mice deficient in both p16 and Mdr2. Mdr2 -/- mice were also treated with fisetin, a flavonoid molecule that selectively kills senescent cells. p16, p21, and inflammatory markers (tumour necrosis factor [TNF]-α, IL-1β, and monocyte chemoattractant protein-1 [MCP-1]) were measured by PCR, and hepatic fibrosis via a hydroxyproline assay and Sirius red staining.
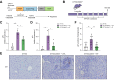
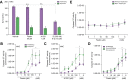
Results: AP20187 treatment reduced p16 and p21 expression by ~35% and ~70% (p >0.05), respectively. Expression of inflammatory markers (TNF-α, IL-1β, and MCP-1) decreased (by 60%, 40%, and 60%, respectively), and fibrosis was reduced by ~60% (p >0.05). Similarly, p16 -/- xMdr2 -/- mice exhibited reduced p21 expression (70%), decreased expression of TNF-α, IL-1β (60%), and MCP-1 (65%) and reduced fibrosis (~50%) (p >0.05) compared with Mdr2 -/- mice. Fisetin treatment reduced expression of p16 and p21 (80% and 90%, respectively), TNF-α (50%), IL-1β (50%), MCP-1 (70%), and fibrosis (60%) (p >0.05).
Conclusions: Our data support a pathophysiological role of cholangiocyte senescence in the progression of PSC, and that targeted removal of senescent cholangiocytes is a plausible therapeutic approach.
Lay summary: Primary sclerosing cholangitis is a fibroinflammatory, incurable biliary disease. We previously reported that biliary epithelial cell senescence (cell-cycle arrest and hypersecretion of profibrotic molecules) is an important phenotype in primary sclerosing cholangitis. Herein, we demonstrate that reducing the number of senescent cholangiocytes leads to a reduction in the expression of inflammatory, fibrotic, and senescence markers associated with the disease.
Keywords: ALP, alkaline phosphatase; AP, AP20187; Apoptosis resistance; BCL2, B cell lymphoma 2; Bcl-xL, B-cell lymphoma-extra large; Biliary epithelial cell; CCA, cholangiocarcinoma; CKI, cyclin-dependent kinase inhibitor; Cellular senescence; Cholestatic liver disease; Col.1A, collagen 1A; D, dasatinib; EVs, extracellular vesicles; FKBP-Casp8, FK506-binding-protein-caspase 8; IF, immunofluorescence; INK-ATTAC, p16Ink4a apoptosis through targeted activation of caspase; IR, irradiation; MCL1, myeloid cell leukemia 1; MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; NHC, normal human cholangiocyte; PSC, primary sclerosing cholangitis; Primary sclerosing cholangitis; Q, quercetin; RT, reverse transcription; SA-β-gal, senescence-associated β-gal; SASP, senescence-associated secretory phenotype; Senescence-associated secretory phenotype; Senolytics; TNF, tumour necrosis factor; WT, wild-type; mdr2, multidrug-resistance 2; qPCR, quantitative PCR; α-SMA, α-smooth muscle actin; β-Gal, β-galactosidase.
© 2021 The Authors.
Conflict of interest statement
The authors have no conflict of interest related to the manuscript. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

