Mucosal-associated invariant T cell responses differ by sex in COVID-19
- PMID: 33870241
- PMCID: PMC8043578
- DOI: 10.1016/j.medj.2021.04.008
Mucosal-associated invariant T cell responses differ by sex in COVID-19
Abstract
Background: Sexual dimorphisms in immune responses contribute to coronavirus disease 2019 (COVID-19) outcomes, but the mechanisms governing this disparity remain incompletely understood.
Methods: We carried out sex-balanced sampling of peripheral blood mononuclear cells from hospitalized and non-hospitalized individuals with confirmed COVID-19, uninfected close contacts, and healthy control individuals for 36-color flow cytometry and single-cell RNA sequencing.
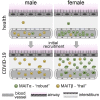
Findings: Our results revealed a pronounced reduction of circulating mucosal-associated invariant T (MAIT) cells in infected females. Integration of published COVID-19 airway tissue datasets suggests that this reduction represented a major wave of MAIT cell extravasation during early infection in females. Moreover, MAIT cells from females possessed an immunologically active gene signature, whereas cells from males were pro-apoptotic.
Conclusions: Our findings uncover a female-specific protective MAIT cell profile, potentially shedding light on reduced COVID-19 susceptibility in females.
Funding: This work was supported by NIH/NIAID (U01AI066569 and UM1AI104681), the Defense Advanced Projects Agency (DARPA; N66001-09-C-2082 and HR0011-17-2-0069), the Veterans Affairs Health System, and Virology Quality Assurance (VQA; 75N93019C00015). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. COVID-19 samples were processed under Biosafety level 2 (BSL-2) with aerosol management enhancement or BSL-3 in the Duke Regional Biocontainment Laboratory, which received partial support for construction from NIH/NIAID (UC6AI058607).
Keywords: IL-7; SARS-CoV-2; apoptosis; innate immunity; monocyte.
© 2021 Elsevier Inc.
Conflict of interest statement
M.T.M. reports grants for biomarker diagnostics from the Defense Advanced Research Projects Agency (DARPA), National Institutes of Health (NIH), Sanofi, and the Department of Veterans Affairs. T.W.B. reports grants from DARPA and is a consultant for Predigen. M.T.M., T.W.B., E.L.T., and C.W.W. report pending patents on molecular methods to diagnose and treat respiratory infections. E.L.T. reports grants on biomarker diagnostics from DARPA and the NIH/Antibacterial Resistance Leadership Group (ARLG) and an ownership stake in Predigen. G.S.G. reports an ownership stake in Predigen. C.W.W. reports grants for biomarker diagnostics from DARPA, NIH/ARLG, Predigen, and Sanofi and has received consultancy fees from bioMerieux, Roche, Biofire, Giner, and Biomeme.
Figures






References
-
- Leong H.N., Earnest A., Lim H.H., Chin C.F., Tan C., Puhaindran M.E., Tan A., Chen M.I., Leo Y.S. SARS in Singapore--predictors of disease severity. Ann. Acad. Med. Singap. 2006;35:326–331. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

