SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system
- PMID: 33870242
- PMCID: PMC8043616
- DOI: 10.1016/j.medj.2021.04.009
SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection appears to increase the risk of adverse pregnancy outcomes, such as pre-eclampsia in pregnant women. The mechanism(s) by which this occurs remains unclear.
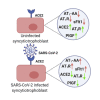
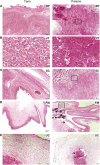
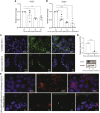
Methods: We investigated the pathophysiology of SARS-CoV-2 at maternal-fetal interface in pregnant women who tested positive for the virus using RNA in situ hybridization (viral RNA), immunohistochemistry, and hematoxylin and eosin staining. To investigate whether viral infection alters the renin angiotensin system (RAS) in placenta, which controls blood pressure, we treated human trophoblasts with recombinant spike protein or a live modified virus with a vesicular stomatitis viral backbone expressing spike protein (VSV-S).
Findings: Viral colonization was highest in maternal decidua, fetal trophoblasts, Hofbauer cells, and in placentas delivered prematurely. We localized SARS-CoV-2 to cells expressing angiotensin-converting enzyme 2 (ACE2) and demonstrate that infected placentas had significantly reduced ACE2. In response to both spike protein and VSV-S, cellular ACE2 decreased although angiotensin II receptor type 1 (AT1R) increased with concomitant increase in soluble fms-like tyrosine kinase-1 (sFlt1). Viral infection decreased pro-angiogenic factors, AT2R, and placental growth factor, which competitively binds to sFlt1. Sera from infected pregnant women had elevated levels of sFlt1 and angiotensin II type 1-receptor autoantibodies prior to delivery, both signatory markers of pre-eclampsia.
Conclusions: SARS-CoV-2 colonizes ACE2-expressing maternal and fetal cells in the placenta. Infection in pregnant women correlates with alteration of placental RAS. As RAS regulates blood pressure, SARS-CoV-2 infection may thus increase adverse hemodynamic outcomes, such as pre-eclampsia in pregnant women.
Funding: NIH/NICHD grants R01 HD091218 and 3R01HD091218-04S1 (RADx-UP Supplement).
Keywords: ACE2; AT1-AA; AT1R; PlGF; decidua; extravillous trophoblasts; placenta; pre-eclampsia; pregnancy; sFlt1.
© 2021 Elsevier Inc.
Conflict of interest statement
I.U.M. serves on the Scientific Advisory Board of Luca Biologics. The authors declare no competing interests.
Figures



References
-
- Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

