Plasma Microbial Cell-free DNA Next-generation Sequencing in the Diagnosis and Management of Febrile Neutropenia
- PMID: 33870413
- PMCID: PMC9070798
- DOI: 10.1093/cid/ciab324
Plasma Microbial Cell-free DNA Next-generation Sequencing in the Diagnosis and Management of Febrile Neutropenia
Abstract
Background: Standard testing fails to identify a pathogen in most patients with febrile neutropenia (FN). We evaluated the ability of the Karius microbial cell-free DNA sequencing test (KT) to identify infectious etiologies of FN and its impact on antimicrobial management.
Methods: This prospective study (ClinicalTrials.gov; NCT02912117) enrolled and analyzed 55 patients with FN. Up to 5 blood samples were collected per subject within 24 hours of fever onset (T1) and every 2 to 3 days. KT results were compared with blood culture (BC) and standard microbiological testing (SMT) results.
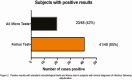
Results: Positive agreement was defined as KT identification of ≥1 isolate also detected by BC. At T1, positive and negative agreement were 90% (9/10) and 31% (14/45), respectively; 61% of KT detections were polymicrobial. Clinical adjudication by 3 independent infectious diseases specialists categorized Karius results as: unlikely to cause FN (N = 0); definite (N = 12): KT identified ≥1 organism also found by SMT within 7 days; probable (N = 19): KT result was compatible with a clinical diagnosis; possible (N = 10): KT result was consistent with infection but not considered a common cause of FN. Definite, probable, and possible cases were deemed true positives. Following adjudication, KT sensitivity and specificity were 85% (41/48) and 100% (14/14), respectively. Calculated time to diagnosis was generally shorter with KT (87%). Adjudicators determined real-time KT results could have allowed early optimization of antimicrobials in 47% of patients, by addition of antibacterials (20%) (mostly against anaerobes [12.7%]), antivirals (14.5%), and/or antifungals (3.6%); and antimicrobial narrowing in 27.3% of cases.
Clinical trials registration: NCT02912117.
Conclusion: KT shows promise in the diagnosis and treatment optimization of FN.
Keywords: Febrile neutropenia; infection; next-generation sequencing.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Sequencing for Infection Diagnostics: Is It Time to embrace the Next Generation?Clin Infect Dis. 2022 May 3;74(9):1669-1670. doi: 10.1093/cid/ciab326. Clin Infect Dis. 2022. PMID: 33870419 No abstract available.
References
-
- Klastersky J. Management of fever in neutropenic patients with different risks of complications. Clin Infect Dis 2004; 39(Suppl 1):S32–7. - PubMed
-
- de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F; ESMO Guidelines Working Group . Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol 2010; 21(Suppl 5):v252–6. - PubMed
-
- Montoya JG, Multani A. Diagnosis of infection in immunocompromised patients: from microscopy to next generation sequencing and host gene signatures. Curr. Opin. Infect. Dis. 2019; 32:295–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

