Probiotics for preventing gestational diabetes
- PMID: 33870484
- PMCID: PMC8094741
- DOI: 10.1002/14651858.CD009951.pub3
Probiotics for preventing gestational diabetes
Abstract
Background: Gestational diabetes mellitus (GDM) is associated with a range of adverse pregnancy outcomes for mother and infant. The prevention of GDM using lifestyle interventions has proven difficult. The gut microbiome (the composite of bacteria present in the intestines) influences host inflammatory pathways, glucose and lipid metabolism and, in other settings, alteration of the gut microbiome has been shown to impact on these host responses. Probiotics are one way of altering the gut microbiome but little is known about their use in influencing the metabolic environment of pregnancy. This is an update of a review last published in 2014.
Objectives: To systematically assess the effects of probiotic supplements used either alone or in combination with pharmacological and non-pharmacological interventions on the prevention of GDM.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (20 March 2020), and reference lists of retrieved studies.
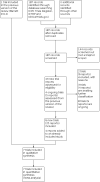
Selection criteria: Randomised and cluster-randomised trials comparing the use of probiotic supplementation with either placebo or diet for the prevention of the development of GDM. Cluster-randomised trials were eligible for inclusion but none were identified. Quasi-randomised and cross-over design studies were not eligible for inclusion in this review. Studies presented only as abstracts with no subsequent full report of study results were only included if study authors confirmed that data in the abstract came from the final analysis. Otherwise, the abstract was left awaiting classification.
Data collection and analysis: Two review authors independently assessed study eligibility, extracted data and assessed risk of bias of included studies. Data were checked for accuracy.
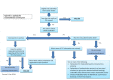
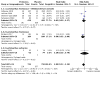
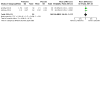
Main results: In this update, we included seven trials with 1647 participants. Two studies were in overweight and obese women, two in obese women and three did not exclude women based on their weight. All included studies compared probiotics with placebo. The included studies were at low risk of bias overall except for one study that had an unclear risk of bias. We excluded two studies, eight studies were ongoing and three studies are awaiting classification. Six included studies with 1440 participants evaluated the risk of GDM. It is uncertain if probiotics have any effect on the risk of GDM compared to placebo (mean risk ratio (RR) 0.80, 95% confidence interval (CI) 0.54 to 1.20; 6 studies, 1440 women; low-certainty evidence). The evidence was low certainty due to substantial heterogeneity and wide CIs that included both appreciable benefit and appreciable harm. Probiotics increase the risk of pre-eclampsia compared to placebo (RR 1.85, 95% CI 1.04 to 3.29; 4 studies, 955 women; high-certainty evidence) and may increase the risk of hypertensive disorders of pregnancy (RR 1.39, 95% CI 0.96 to 2.01, 4 studies, 955 women), although the CIs for hypertensive disorders of pregnancy also indicated probiotics may have no effect. There were few differences between groups for other primary outcomes. Probiotics make little to no difference in the risk of caesarean section (RR 1.00, 95% CI 0.86 to 1.17; 6 studies, 1520 women; high-certainty evidence), and probably make little to no difference in maternal weight gain during pregnancy (MD 0.30 kg, 95% CI -0.67 to 1.26; 4 studies, 853 women; moderate-certainty evidence). Probiotics probably make little to no difference in the incidence of large-for-gestational age infants (RR 0.99, 95% CI 0.72 to 1.36; 4 studies, 919 infants; moderate-certainty evidence) and may make little to no difference in neonatal adiposity (2 studies, 320 infants; data not pooled; low-certainty evidence). One study reported adiposity as fat mass (MD -0.04 kg, 95% CI -0.12 to 0.04), and one study reported adiposity as percentage fat (MD -0.10%, 95% CI -1.19 to 0.99). We do not know the effect of probiotics on perinatal mortality (RR 0.33, 95% CI 0.01 to 8.02; 3 studies, 709 infants; low-certainty evidence), a composite measure of neonatal morbidity (RR 0.69, 95% CI 0.36 to 1.35; 2 studies, 623 infants; low-certainty evidence), or neonatal hypoglycaemia (mean RR 1.15, 95% CI 0.69 to 1.92; 2 studies, 586 infants; low-certainty evidence). No included studies reported on perineal trauma, postnatal depression, maternal and infant development of diabetes or neurosensory disability.
Authors' conclusions: Low-certainty evidence from six trials has not clearly identified the effect of probiotics on the risk of GDM. However, high-certainty evidence suggests there is an increased risk of pre-eclampsia with probiotic administration. There were no other clear differences between probiotics and placebo among the other primary outcomes. The certainty of evidence for this review's primary outcomes ranged from low to high, with downgrading due to concerns about substantial heterogeneity between studies, wide CIs and low event rates. Given the risk of harm and little observed benefit, we urge caution in using probiotics during pregnancy. The apparent effect of probiotics on pre-eclampsia warrants particular consideration. Eight studies are currently ongoing, and we suggest that these studies take particular care in follow-up and examination of the effect on pre-eclampsia and hypertensive disorders of pregnancy. In addition, the underlying potential physiology of the relationship between probiotics and pre-eclampsia risk should be considered.
Trial registration: ClinicalTrials.gov NCT00167700 NCT01922791 NCT02692820 NCT01436448 NCT04009889.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SJD: none.
HB: recieved a grant from the NHMRC, Australia (a competitive government research grant) to undertake a randomised control trial of probiotics for the prevention of gestational diabetes mellitus and I was an associate investigator this trial (Callaway 2019). In this review, HB was not involved in any decisions relating to this trial: assessment of the trial for inclusion, assessment of risk of bias and data extraction were carried out by individuals who were not directly involved in the trial. SJD and SAP carried out these tasks. Chr. Hansen A/S have donated the probiotics and matching placebo to the SPRING study (Callaway 2019) that was conducted by authors Barrett, Dekker Nitert and Callaway. While the authors are grateful for this donation from Chr.Hansen A/S, the conduct of the study, analysis and publication of the results is entirely independent of Chr. Hansen A/S.
SAP: none.
LC: was chief investigator in a trial examining the use of probiotics for preventing gestational diabetes mellitus (Callaway 2019), which was funded from a grant from the NHMRC, Australia (a competitive government research grant). In this review, LC was not involved in any decisions relating to this trial: assessment of the trial for inclusion, assessment of risk of bias and data extraction were carried out by individuals who were not directly involved in the trial. SJD and SAP carried out these tasks. Chr. Hansen A/S have donated the probiotics and matching placebo to the SPRING study (Callaway 2019) that was conducted by authors Barrett, Dekker Nitert and Callaway. While the authors are grateful for this donation from Chr.Hansen A/S, the conduct of the study, analysis and publication of the results is entirely independent of Chr. Hansen A/S.
MDN: was scientific lead in a trial examining the use of probiotics for preventing gestational diabetes mellitus (Callaway 2019), which was funded from a grant from the NHMRC, Australia (a competitive government research grant). In this review, MDN was not involved in any decisions relating to this trial: assessment of the trial for inclusion, assessment of risk of bias and data extraction were carried out by individuals who were not directly involved in the trial. SJD and SAP carried out these tasks. Chr. Hansen A/S have donated the probiotics and matching placebo to the SPRING study (Callaway 2019) that was conducted by authors Barrett, Dekker Nitert and Callaway. While the authors are grateful for this donation from Chr.Hansen A/S, the conduct of the study, analysis and publication of the results is entirely independent of Chr. Hansen A/S.
Figures


















































Update of
-
Probiotics for preventing gestational diabetes.Cochrane Database Syst Rev. 2014 Feb 27;2014(2):CD009951. doi: 10.1002/14651858.CD009951.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD009951. doi: 10.1002/14651858.CD009951.pub3. PMID: 24574258 Free PMC article. Updated.
Comment in
-
Probiotics for preventing gestational diabetes: Summary of a Cochrane Review.Explore (NY). 2021 Nov-Dec;17(6):590-591. doi: 10.1016/j.explore.2021.08.005. Epub 2021 Sep 1. Explore (NY). 2021. PMID: 34526233 No abstract available.
References
References to studies included in this review
Callaway 2019 {published data only}ACTRN12611001208998
-
- Callaway L. Randomized placebo controlled trial of probiotics in overweight and obese women to assess the prevention of gestational diabetes. www.anzctr.org.au/trial_view.aspx?ID=347738 (accessed 6 July 2012).
-
- Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care 2019;42(3):364-71. [CENTRAL: CN-01915884] [EMBASE: 2001611803] [PMID: ] - PubMed
Jamilian 2016 {published data only}IRCT201503035623N38
-
- IRCT201503035623N38. Effect of probiotic in the prevention of spontaneously abortion. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201503035623N38 2015. [CENTRAL: CN-01833874]
-
- Jamilian M, Bahmani F, Vahedpoor Z, Salmani A, Tajabadi-Ebrahimi M, Jafari P, et al. Effects of probiotic supplementation on metabolic status in pregnant women: a randomized, double-blind, placebo-controlled trial. Archives of Iranian Medicine 2016;19(10):687-92. [CENTRAL: CN-01307046] [PMID: ] - PubMed
Laitinen 2009 {published data only}
-
- Aaltonen J, Ojala T, Laitinen K, Piirainen TJ, Poussa TA, Isolauri E. Evidence of infant blood pressure programming by maternal nutrition during pregnancy: a prospective randomized controlled intervention study. Journal of Pediatrics 2008;152:79-84. - PubMed
-
- Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled study. European Journal of Clinical Nutrition 2011;65(1):10-9. - PubMed
-
- Hoppu U, Isolauri E, Koskinen P, Laitinen K. Diet and blood lipids in 1-4 year-old children. Nutrition Metabolism & Cardiovascular Diseases 2013;23(10):980-6. - PubMed
-
- Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2011;30(2):156-64. [CENTRAL: CN-00786714] [PMID: ] - PubMed
Lindsay 2014 {published data only}ISRCTN97241163
-
- Lindsay K, Kennelly M, Smith T, Maguire O, Shanahan F, Brennan L, et al. Probiotics in obese pregnancy to reduce maternal fasting glucose: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A156. - PubMed
-
- Lindsay K, Maguire O, Smith T, Brennan L, McAuliffe F. A randomized controlled trial of probiotics to reduce maternal glycaemia in obese pregnancy. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S342.
-
- Lindsay KL, Brennan L, McAuliffe FM. Acceptability of and compliance with a probiotic capsule intervention in pregnancy. International Journal of Gynecology and Obstetrics 2014;125(3):279-80. - PubMed
-
- Lindsay KL, Brennan L, McAuliffe FM. Probiotics in obese pregnancy to reduce maternal fasting glucose: a randomized controlled trial. The Power of Programming 2014: International Conference on Developmental Origins of Adiposity and Long-term Health; 2014 Mar 13-15; Munich, Germany.
-
- Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial. American Journal of Clinical Nutrition 2014;99:1432-9. - PubMed
Okesene‐Gafa 2019 {published and unpublished data}ACTRN12615000400561
-
- ACTRN12615000400561. A four-armed randomised controlled demonstration trial of a multifaceted dietary intervention and probiotic capsules in obese pregnant women in the Counties Manukau Health region. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12615000400561 (first received 10 April 2015). [CENTRAL: CN-02089252]
-
- McKinlay C, Okesene-Gafa K, Taylor R, Wall C, Rush E, McCowan M, et al. Dietary intervention and/or probiotic capsules in obese pregnant women and infant growth and feeding at 5 months: healthy mums and babies (HUMBA) trial. Journal of Paediatrics and Child Health 2019;55:35. [CENTRAL: CN-01940481] [EMBASE: 627192706]
-
- Okesene-Gafa K, Li M, Taylor RS, Thompson JM, Crowther CA, McKinlay CJ, et al. A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial. BMC Pregnancy and Childbirth 2016;16(1):373. [CENTRAL: CN-01426401] [EMBASE: 613355126] [PMCID: PMC5123375] [PMID: ] - PMC - PubMed
-
- Okesene-Gafa K, Li M, Taylor RS, Thompson JM, Crowther CA, McKinlay CJ, et al. Correction: a randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial. BMC Pregnancy and Childbirth 2018;18(1):130. [CENTRAL: CN-01607638] [EMBASE: 621978171] [PMID: ] - PMC - PubMed
Pellonpera 2019 {published data only}
-
- Laitinen K. Nutrition and pregnancy intervention study. clinicaltrials.gov/ct2/show/NCT01922791 (first received 14 August 2013).
-
- Pellonpera O, Mokkala K, Houttu N, Vahlberg T, Koivuniemi E, Tertti K, et al. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care 2019;42(6):1009-17. [CENTRAL: CN-01963280] [EMBASE: 2002045489] [PMID: ] - PubMed
Wickens 2017 {published data only}ACTRN12612000196842
-
- Barthow C, Wickens K, Stanley T, Mitchell EA, Maude R, Abels P, et al. The Probiotics in Pregnancy Study (PiP study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy and Childbirth 2016;16(1):133. [CENTRAL: CN-01425048] [EMBASE: 20160469757] [PMCID: PMC4891898] [PMID: ] - PMC - PubMed
-
- Maude R, Barthow C, Wickens K, Murphy R, Abels P, Stone P, et al. Can early pregnancy probiotic supplementation reduce the rate of gestational diabetes? In: 31st International Confederation of Midwives Triennial Congress. Midwives – Making a Difference in the World; 2017 Jun 18-22; Toronto, Canada. 2017:P1.089. [CENTRAL: CN-02082804]
-
- Wickens K, Barthow C, Mitchell EA, Stanley TV, Purdie G, Rowden J, et al. Maternal supplementation alone with lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatric Allergy and Immunology 2018;29(3):296-302. [CENTRAL: CN-01608243] [EMBASE: 621550781] [PMID: ] - PubMed
-
- Wickens K. A randomized placebo controlled trial of the effects of the probiotic Lactobacillus rhamnosus HN001 taken from the 1st trimester of pregnancy till 6 months post partum, if breastfeeding, on the development of eczema and atopic sensitization in infants by age 12 months. www.anzctr.org.au/trial_view.aspx?ID=362049 (first received 7 February 2012).
-
- Wickens KL, Barthow CA, Murphy R, Abels PR, Maude RM, Stone PR, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. British Journal of Nutrition 2017;117(6):804-13. [CENTRAL: CN-01381612] [EMBASE: 615245454] [PMID: ] - PMC - PubMed
References to studies excluded from this review
Asemi 2013 {published data only}
-
- Asemi Z, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: a randomized controlled trial. Pakistan Journal of Biological Sciences : PJBS 2011;14(8):476-82. [CENTRAL: CN-00798130] [PMID: ] - PubMed
-
- Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. Journal of Maternal-fetal & Neonatal Medicine 2012;25(9):1552-6. [CENTRAL: CN-00971455] [PMID: ] - PubMed
-
- Asemi Z, Samimi M, Tabassi Z, Naghibi M, Rahimi A, Khorammian H, et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. European Journal of Clinical Nutrition 2013;67(1):71-4. - PubMed
-
- IRCT138811282394N3. Comparison of effects of consumption of probiotic and conventional yogurt on oxidative stress and serum TNF-α and CRP in pregnant women and anthropometric indices of neonates. www.irct.ir/trial/2075 (first received 6 June 2011). [CENTRAL: CN-01804915]
Taghizadeh 2014 {published data only}
-
- Taghizadeh M, Asemi Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: a randomized controlled clinical trial. Hormones 2014;13(3):398-406. [CENTRAL: CN-00999375] [EMBASE: 2014487933] [PMID: ] - PubMed
References to studies awaiting assessment
Asgharian 2020 {published data only}IRCT201604013706N31
-
- Asgharian H, Homayouni-Rad A, Mirghafourvand M, Mohammad-Alizadeh-Charandabi S. Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: a randomized controlled clinical trial. European Journal of Nutrition 2020;59(1):205-15. [CENTRAL: CN-01936694] [EMBASE: 627693578] [PMID: ] - PubMed
-
- IRCT201604013706N31. Effect of probiotic yoghurt on level of blood glucose in obese and overweight pregnant women. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201604013706N31 2016. [CENTRAL: CN-01856226]
Charles 2018 {published data only}
-
- Charles D, Drymoussi Z, Thangaratinam S. The effect of probiotic supplementation on gestational diabetes mellitus and metabolic outcomes: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2018;125:10. [CENTRAL: CN-01713894] [EMBASE: 625506633]
References to ongoing studies
ACTRN12620000359932 {published data only}
-
- ACTRN12620000359932. Probiotics in the prevention of gestational diabetes mellitus in women at increased risk: a prospective randomised controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000359932 (first received 28 January 2020).
Godfrey 2017 {published data only}
-
- Godfrey KM, Cutfield W, Chan S-Y, Baker PN, Chong Y-S, Aris IBM, et al. Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health ("NiPPeR"): study protocol for a randomised controlled trial. Trials 2017;18:131. [CENTRAL: CN-01454636] [EMBASE: 614884778] [PMID: ] - PMC - PubMed
Halkjaer 2016 {published data only}
-
- NCT02508844. Effect of probiotics (Vivomixx) on weight, microbiota and glucose tolerance in obese pregnant women and their newborn. clinicaltrials.gov/show/NCT02508844 (first received 27 July 2015). [CENTRAL: CN-02032725]
IRCT20161025030502N2 {published data only}IRCT20161025030502N2
-
- IRCT20161025030502N2. Effect of oral probiotic lactofem on metabolic parameters in overweight pregnant women referred to prenatal clinics of the shiraz hospitals in 2016. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20161025030502N2 2016. [CENTRAL: CN-01896627]
IRCT20180509039595N1 {published data only}
-
- IRCT20180509039595N1. The effect of probiotic capsule on prevention of gestational diabetes. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180509039595N1 2018. [CENTRAL: CN-01907075]
NCT01436448 {published data only}
-
- NCT01436448. Effects of probiotics (Lactobacillus rhamnosus) in reducing glucose intolerance during and after pregnancy: a double blind randomized controlled trial in antenatal clinic of Karachi-Pakistan (GRIP). clinicaltrials.gov/show/NCT01436448 (first received 19 September 2011).
NCT03240419 {published data only}
-
- NCT03240419. Prenatal probiotic intervention. clinicaltrials.gov/show/NCT03240419 (first received 7 August 2017). [CENTRAL: CN-02089253]
NCT04009889 {published data only}
-
- NCT04009889. Impact of oral probiotic blend on pregnancy outcome. clinicaltrials.gov/show/NCT04009889 (first received 5 July 2019). [CENTRAL: CN-01953073]
Additional references
Abouzeid 2014
ADA 2019
-
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2019. Diabetes Care 2019;42(Suppl 1):S13-S28. - PubMed
Agha‐Jaffar 2016
-
- Agha-Jaffar R, Oliver N, Johnston D, Robinson S. Gestational diabetes mellitus: does an effective prevention strategy exist? Nature Reviews. Endocrinology 2016;12(9):533-46. - PubMed
Barrett 2012
-
- Barrett HL, Callaway LK, Nitert MD. Probiotics: a potential role in the prevention of gestational diabetes. Acta Diabetologia 2012;49(Suppl 1):S1-S13. - PubMed
Brunkwall 2017
Buchanan 2012
Carlisle 2017
-
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. - PubMed
Carpenter 1982
-
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. American Journal of Obstetrics and Gynecology 1982;144(7):768-73. - PubMed
Carr 2011
-
- Carr DB, Newton KM, Utzschneider KM, Faulenbach MV, Kahn SE, Easterling TR, et al. Gestational diabetes or lesser degrees of glucose intolerance and risk of preeclampsia. Hypertension in Pregnancy 2011;30(2):153-63. - PubMed
Chatzakis 2019
-
- Chatzakis C, Goulis DG, Mareti E, Eleftheriades M, Zavlanos A, Dinas K, et al. Prevention of gestational diabetes mellitus in overweight or obese pregnant women: a network meta-analysis. Diabetes Research and Clinical Practice 2019;158:107924. - PubMed
Cho 2018
-
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice 2018;138:271-81. - PubMed
Crawford 2015
Crowther 2005
-
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477-86. - PubMed
Crusell 2018
Dabelea 2005
-
- Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005;28(3):579-84. - PubMed
David 2014
Dodd 2007
-
- Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2007;47(4):307-12. - PubMed
Esakoff 2009
-
- Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 2009;200(6):672 e1-4. - PubMed
Facchinetti 2014
-
- Facchinetti F, Dante G, Petrella E, Neri I. Dietary interventions, lifestyle changes, and dietary supplements in preventing gestational diabetes mellitus: a literature review. Obstetrical & Gynecological Survey 2014;69(11):669-80. - PubMed
FAO/WHO 2006
-
- Food and Agriculture Organization of the United Nations, World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. http://www.fao.org/3/a0512e/a0512e.pdf 2006;accessed January 2021.
Fitria 2019
Gomez Arango 2015
-
- Gomez Arango LF, Barrett HL, Callaway LK, Nitert MD. Probiotics and pregnancy. Current Diabetes Reports 2015;15(1):567. - PubMed
GRADE Handbook
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available at gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed January 2021. Available at gradepro.org.
Griffin 2015
-
- Griffin C. Probiotics in obstetrics and gynaecology. Australian & New Zealand Journal of Obstetrics & Gynaecology 2015;55(3):201-9. - PubMed
Griffith 2020
-
- Griffith R J, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD012394. [DOI: 10.1002/14651858.CD012394.pub3] - DOI - PMC - PubMed
Han 2012
HAPO 2008
-
- The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358:1991-2002. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
IADPSG 2010
-
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676-82. - PMC - PubMed
Jarde 2018
John 2016
-
- John GK, Mullin GE. The gut microbiome and obesity. Current Oncology Reports 2016;18(7):45. - PubMed
Koren 2012
Landon 2009
Lindsay 2013
-
- Lindsay KL, Walsh CA, Brennan L, McAuliffe FM. Probiotics in pregnancy and maternal outcomes: a systematic review. Journal of Maternal Fetal and Neonatal Medicine 2013;26(8):772-8. - PubMed
López‐de‐Andrés 2020
-
- López-de-Andrés A, Perez-Farinos N, Hernández-Barrera V, Palomar-Gallego MA, Carabantes-Alarcón D, Zamorano-León JJ, Miguel-Diez J, Jimenez-Garcia R. A Population-Based Study of Diabetes During Pregnancy in Spain (2009-2015): Trends in Incidence, Obstetric Interventions, and Pregnancy Outcomes.. Journal of Clinical Medicine 2020;9(2):582. - PMC - PubMed
Malcolm 2012
-
- Malcolm J. Through the looking glass: gestational diabetes as a predictor of maternal and offspring long-term health. Diabetes/Metabolism Research and Reviews 2012;28(4):307-11. - PubMed
Masulli 2020
-
- Masulli M, Vitacolonna E, Fraticelli F, Della Pepa G, Mannucci E, Monami M. Effects of probiotic supplementation during pregnancy on metabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Research and Clinical Practice 2020;162:108111. - PubMed
Metzger 1998
-
- Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998;21 Suppl 2:B161-7. - PubMed
Ministry of Health 2014
-
- Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: a Clinical Practice Guideline. Wellington (NZ): Ministry of Health, 2014.
Musso 2011
-
- Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annual Review of Medicine 2011;62:361-80. - PubMed
Noh 2021
Okesene‐Gafa 2020
-
- Okesene-Gafa KA, Moore AE, Jordan V, McCowan L, Crowther CA. Probiotic treatment for women with gestational diabetes to improve maternal and infant health and well-being. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD012970. [DOI: 10.1002/14651858.CD012970.pub2] - DOI - PMC - PubMed
Plows 2019
-
- Plows JF, Reynolds CM, Vickers MH, Baker PN, Stanley JL. Nutritional supplementation for the prevention and/or treatment of gestational diabetes mellitus. Current Diabetes Reports 2019;19(9):73. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogozinska 2015
Shepherd 2017
Simmons 2015
-
- Simmons D. Prevention of gestational diabetes mellitus: where are we now? Diabetes, Obesity & Metabolism 2015;17(9):824-34. - PubMed
Sui 2013
-
- Sui Z, Turnbull DA, Dodd JM. Overweight and obese women's perceptions about making healthy change during pregnancy: a mixed method study. Maternal and Child Health Journal 2013;17(10):1879-87. - PubMed
Syngai 2016
The Finnish Medical Society Duodecim 2013
-
- Duodecim of the Finnish Medical Association, the Medical Council of the Finnish Diabetes Association and the Finnish Gynecological Association. Gestational Diabetes: Current Care Guidelines. Helsinki (Finland): The Finnish Medical Society Duodecim, 2013.
Tiderencel 2020
-
- Tiderencel KA, Hutcheon DA, Ziegler J. Probiotics for the treatment of type 2 diabetes: a review of randomized controlled trials. Diabetes/Metabolism Research and Reviews 2020;36(1):e3213. - PubMed
Tieu 2017
van de Vusse 2013
-
- de Vusse L, Hanson L, Safdar N. Perinatal outcomes of prenatal probiotic and prebiotic administration: an integrative review. Journal of Perinatal & Neonatal Nursing 2013;27(4):288-301. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

