A meta-analysis of the activity, stability, and mutational characteristics of temperature-adapted enzymes
- PMID: 33871022
- PMCID: PMC8150157
- DOI: 10.1042/BSR20210336
A meta-analysis of the activity, stability, and mutational characteristics of temperature-adapted enzymes
Abstract
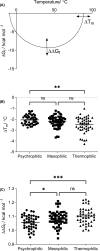
Understanding the characteristics that define temperature-adapted enzymes has been a major goal of extremophile enzymology in recent decades. In the present study, we explore these characteristics by comparing psychrophilic, mesophilic, and thermophilic enzymes. Through a meta-analysis of existing data, we show that psychrophilic enzymes exhibit a significantly larger gap (Tg) between their optimum and melting temperatures compared with mesophilic and thermophilic enzymes. These results suggest that Tg may be a useful indicator as to whether an enzyme is psychrophilic or not and that models of psychrophilic enzyme catalysis need to account for this gap. Additionally, by using predictive protein stability software, HoTMuSiC and PoPMuSiC, we show that the deleterious nature of amino acid substitutions to protein stability increases from psychrophiles to thermophiles. How this ultimately affects the mutational tolerance and evolutionary rate of temperature adapted organisms is currently unknown.
Keywords: enzyme activity; enzymology; extremophiles; mutation; protein stability.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
-
- Feller G. and Gerday C. (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat. Rev. Microbiol. 1, 200–208 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

