Modulating skin colour: role of the thioredoxin and glutathione systems in regulating melanogenesis
- PMID: 33871027
- PMCID: PMC8112849
- DOI: 10.1042/BSR20210427
Modulating skin colour: role of the thioredoxin and glutathione systems in regulating melanogenesis
Abstract
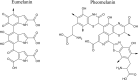
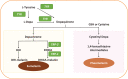
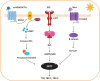
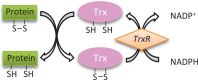
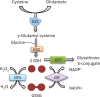
Different skin colour among individuals is determined by the varying amount and types of melanin pigment. Melanin is produced in melanocytes, a type of dendritic cell located in the basal layer of the epidermis, through the process of melanogenesis. Melanogenesis consists of a series of biochemical and enzymatic reactions catalysed by tyrosinase and other tyrosinase-related proteins, leading to the formation of two types of melanin, eumelanin and pheomelanin. Melanogenesis can be regulated intrinsically by several signalling pathways, including the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA), stem cell factor (SCF)/c-kit and wingless-related integration site (Wnt)/β-catenin signalling pathways. Ultraviolet radiation (UVR) is the major extrinsic factor in the regulation of melanogenesis, through the generation of reactive oxygen species (ROS). Antioxidants or antioxidant systems, with the ability to scavenge ROS, may decrease melanogenesis. This review focuses on the two main cellular antioxidant systems, the thioredoxin (Trx) and glutathione (GSH) systems, and discusses their roles in melanogenesis. In the Trx system, high levels/activities of thioredoxin reductase (TrxR) are correlated with melanin formation. The GSH system is linked with regulating pheomelanin formation. Exogenous addition of GSH has been shown to act as a depigmenting agent, suggesting that other antioxidants may also have the potential to act as depigmenting agents for the treatment of human hyperpigmentation disorders.
Keywords: antioxidants; glutathione; hyperpigmentation; melanogenesis; thioredoxin.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

