Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): a position statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET)
- PMID: 33871350
- PMCID: PMC8297668
- DOI: 10.2450/2021.0117-21
Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): a position statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET)
Figures
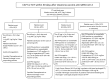
References
-
- The BMJ [internet] Covid-19: EMA defends AstraZeneca vaccine as Germany and Canada halt rollouts. BMJ. 2021. [Accessed on 09/04/2021.]. Available at: https://www.bmj.com/content/373/bmj.n883/rapid-responses. - PubMed
-
- European Medicines Agency (EMA) [internet] COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. [Accessed on 09/04/2021.]. Available at: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
