Association of US Food and Drug Administration Removal of Indications for Use of Oral Quinolones With Prescribing Trends
- PMID: 33871571
- PMCID: PMC8056313
- DOI: 10.1001/jamainternmed.2021.1154
Association of US Food and Drug Administration Removal of Indications for Use of Oral Quinolones With Prescribing Trends
Abstract
Importance: In May 2016, due to concerns of the risks outweighing the benefits, the US Food and Drug Administration (FDA) removed systemic quinolones' indications for acute, uncomplicated urinary tract infection (uUTI), acute sinusitis (AS), and acute exacerbation of chronic obstructive pulmonary disease (AE-COPD). How the change influenced oral quinolone use is unknown.
Objective: To assess the association of oral quinolone safety warnings and indication restrictions with use.
Design, setting, and participants: This interrupted time series (January 2015-November 2018) analysis of the monthly prevalence of oral quinolone-treated infection episodes used a national sample of privately insured patients in outpatient care from the IBM MarketScan Database and included adults with antibiotic treatment of new uUTI, AS, or AE-COPD episodes, excluding patients with conditions that complicate infections, previous hospitalization, or other infections.
Exposures: Time before and after May 2016 when the FDA mandated label changes.
Main outcomes and measures: Monthly oral quinolone use prevalence by each condition before and after the label changes, overall and stratified by prescriber specialty.
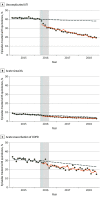
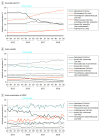
Results: In January 2015, quinolone prevalence among antibiotic-treated uUTI episodes (n = 652 235) was 41.6% (95% CI, 40.6%-42.5%); AS (n = 1 742 248) was 8.3% (95% CI, 7.9%-8.6%), and AE-COPD (n = 22 817) was 31.9% (95% CI, 30.3%-33.4%). Before the label changes, trends in monthly quinolone prevalence were nearly flat. The month of the label changes we noted an immediate reduction for uUTI (-7.2%; 95% CI, -8.6% to -5.8%); and to a lesser extent for AS (-1.2%; 95% CI, -1.5% to -0.9%) and AE-COPD (-2.6%; 95% CI, -4.1% to -1.1%), and continued monthly declines thereafter. Falsification tests confirmed an immediate decrease after the label change of quinolone use for uUTI but more obscured effects for AS and AE-COPD. Treatment shifted mostly to first-line (eg, nitrofurantoin in uUTI, amoxicillin in AS, macrolides in AE-COPD) and other second-line agents but use of not recommended antibiotics also increased (eg, tetracyclines in AE-COPD). Prescribing preferences varied, but significant reductions were seen across all prescriber specialties. At the end of the study period, quinolone was used for 19.2% of treated uUTIs, 2.9% of treated AS, and 14.6% of treated AE-COPD episodes.
Conclusions and relevance: Label changes and their announcements was associated with an immediate reduction in oral quinolone use for uUTI and to a lesser extent for AS and AE-COPD. Quinolones continued to contribute a considerable proportion of treatments for uUTI and AE-COPD episodes at the end of the study period, pointing to opportunities for further improvement.
Conflict of interest statement
Figures
References
-
- Pitiriga V, Vrioni G, Saroglou G, Tsakris A. The impact of antibiotic stewardship programs in combating quinolone resistance: a systematic review and recommendations for more efficient interventions. Advances in Therapy. 2017;34(4):854-865. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

