Informing Women About Overdetection in Breast Cancer Screening: Two-Year Outcomes From a Randomized Trial
- PMID: 33871631
- PMCID: PMC8562961
- DOI: 10.1093/jnci/djab083
Informing Women About Overdetection in Breast Cancer Screening: Two-Year Outcomes From a Randomized Trial
Abstract
Background: Supporting well-informed decisions about breast cancer screening requires communicating that inconsequential disease may be detected, leading to overdiagnosis and overtreatment. Having previously shown that telling women about overdetection improved informed choice, we investigated effects on screening knowledge and participation over 2 years.
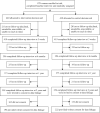
Methods: We conducted a community-based, parallel-group, randomized controlled trial in Australia. Participants were women aged 48-50 years, without personal or strong family history of breast cancer, who had not undergone mammography in the past 2 years. We randomly assigned 879 women to receive the intervention decision aid (evidence-based information on overdetection, breast cancer mortality reduction, and false-positives) or control decision aid (identical but without overdetection information). We interviewed 838 women postintervention and recontacted them for follow-up at 6 months and 1 and 2 years. Main outcomes for this report are screening knowledge and participation.
Results: We interviewed 790, 746, and 712 participants at 6 months, 1, and 2 years, respectively. The intervention group demonstrated superior knowledge throughout follow-up. After 2 years, conceptual knowledge was adequate in 123 (34.4%) of 358 women in the intervention group compared with 71 (20.1%) of 354 control participants(odds ratio = 2.04, 95% confidence interval = 1.46 to 2.85). Groups were similar in total screening participation (200 [55.1%] vs 204 [56.0%]; = 0.97, 95% confidence interval = 0.73 to 1.29).
Conclusions: A brief decision aid produced lasting improvement in women's understanding of potential consequences of screening, including overdetection, without changing participation rates. These findings support the use of decision aids for breast cancer screening.
© The Author(s) 2021. Published by Oxford University Press.
Figures

Comment in
-
RE: Informing women about overdetection in breast cancer screening: Two-year outcomes from a randomized trial.J Natl Cancer Inst. 2023 Jan 10;115(1):112-113. doi: 10.1093/jnci/djac200. J Natl Cancer Inst. 2023. PMID: 36331345 Free PMC article. No abstract available.
References
-
- de Ligt KM, Heins M, Verloop J. , et al. Patient-reported health problems and healthcare use after treatment for early-stage breast cancer. Breast. 2019;46:4–11. - PubMed
-
- Taylor C, Correa C, Duane FK. , et al. ; for the Early Breast Cancer Trialists’ Collaborative Group. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–1649. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

