Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50
- PMID: 33872070
- PMCID: PMC8280089
- DOI: 10.1200/JCO.21.00174
Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50
Abstract
Purpose: We report the first 5-year follow-up of any first-line phase III immunotherapy trial for non-small-cell lung cancer (NSCLC). KEYNOTE-024 (ClinicalTrials.gov identifier: NCT02142738) is an open-label, randomized controlled trial of pembrolizumab compared with platinum-based chemotherapy in patients with previously untreated NSCLC with a programmed death ligand-1 (PD-L1) tumor proportion score of at least 50% and no sensitizing EGFR or ALK alterations. Previous analyses showed pembrolizumab significantly improved progression-free survival and overall survival (OS).
Methods: Eligible patients were randomly assigned (1:1) to pembrolizumab (200 mg once every 3 weeks for up to 35 cycles) or platinum-based chemotherapy. Patients in the chemotherapy group with progressive disease could cross over to pembrolizumab. The primary end point was progression-free survival; OS was a secondary end point.
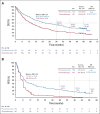
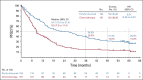
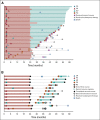
Results: Three hundred five patients were randomly assigned: 154 to pembrolizumab and 151 to chemotherapy. Median (range) time from randomization to data cutoff (June 1, 2020) was 59.9 (55.1-68.4) months. Among patients initially assigned to chemotherapy, 99 received subsequent anti-PD-1 or PD-L1 therapy, representing a 66.0% effective crossover rate. Median OS was 26.3 months (95% CI, 18.3 to 40.4) for pembrolizumab and 13.4 months (9.4-18.3) for chemotherapy (hazard ratio, 0.62; 95% CI, 0.48 to 0.81). Kaplan-Meier estimates of the 5-year OS rate were 31.9% for the pembrolizumab group and 16.3% for the chemotherapy group. Thirty-nine patients received 35 cycles (ie, approximately 2 years) of pembrolizumab, 82.1% of whom were still alive at data cutoff (approximately 5 years). Toxicity did not increase with longer treatment exposure.
Conclusion: Pembrolizumab provides a durable, clinically meaningful long-term OS benefit versus chemotherapy as first-line therapy for metastatic NSCLC with PD-L1 tumor proportion score of at least 50%.
Conflict of interest statement
Figures
Comment in
-
Paving the Way for Long-Term Survival in Non-Small-Cell Lung Cancer.J Clin Oncol. 2021 Jul 20;39(21):2321-2323. doi: 10.1200/JCO.21.00760. Epub 2021 Jun 8. J Clin Oncol. 2021. PMID: 34101497 No abstract available.
References
-
- Howlader N Noone AM Krapcho M, et al. : SEER Cancer Statistics Review, 1975-2014. Bethesda, MD, National Cancer Institute, 2017
-
- Butler CA Darragh KM Currie GP, et al. : Variation in lung cancer survival rates between countries: Do differences in data reporting contribute? Respir Med 100:1642-1646, 2006 - PubMed
-
- National Comprehensive Cancer Network (NCCN) : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-small Cell Lung Cancer. Version 2.2020. https://www.nccn.org
-
- Reck M Rodriguez-Abreu D Robinson AG, et al. : Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37:537-546, 2019 - PubMed
-
- Mok TSK Wu YL Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-1830, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

