Returning to a Normal Life via COVID-19 Vaccines in the United States: A Large-scale Agent-Based Simulation Study
- PMID: 33872188
- PMCID: PMC8086790
- DOI: 10.2196/27419
Returning to a Normal Life via COVID-19 Vaccines in the United States: A Large-scale Agent-Based Simulation Study
Abstract
Background: In 2020, COVID-19 has claimed more than 300,000 deaths in the United States alone. Although nonpharmaceutical interventions were implemented by federal and state governments in the United States, these efforts have failed to contain the virus. Following the Food and Drug Administration's approval of two COVID-19 vaccines, however, the hope for the return to normalcy has been renewed. This hope rests on an unprecedented nationwide vaccine campaign, which faces many logistical challenges and is also contingent on several factors whose values are currently unknown.
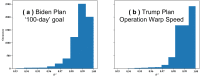
Objective: We study the effectiveness of a nationwide vaccine campaign in response to different vaccine efficacies, the willingness of the population to be vaccinated, and the daily vaccine capacity under two different federal plans. To characterize the possible outcomes most accurately, we also account for the interactions between nonpharmaceutical interventions and vaccines through 6 scenarios that capture a range of possible impacts from nonpharmaceutical interventions.
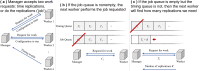
Methods: We used large-scale, cloud-based, agent-based simulations by implementing the vaccination campaign using COVASIM, an open-source agent-based model for COVID-19 that has been used in several peer-reviewed studies and accounts for individual heterogeneity and a multiplicity of contact networks. Several modifications to the parameters and simulation logic were made to better align the model with current evidence. We chose 6 nonpharmaceutical intervention scenarios and applied the vaccination intervention following both the plan proposed by Operation Warp Speed (former Trump administration) and the plan of one million vaccines per day, proposed by the Biden administration. We accounted for unknowns in vaccine efficacies and levels of population compliance by varying both parameters. For each experiment, the cumulative infection growth was fitted to a logistic growth model, and the carrying capacities and the growth rates were recorded.
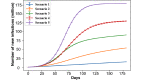
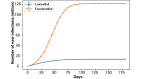
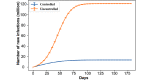
Results: For both vaccination plans and all nonpharmaceutical intervention scenarios, the presence of the vaccine intervention considerably lowers the total number of infections when life returns to normal, even when the population compliance to vaccines is as low as 20%. We noted an unintended consequence; given the vaccine availability estimates under both federal plans and the focus on vaccinating individuals by age categories, a significant reduction in nonpharmaceutical interventions results in a counterintuitive situation in which higher vaccine compliance then leads to more total infections.
Conclusions: Although potent, vaccines alone cannot effectively end the pandemic given the current availability estimates and the adopted vaccination strategy. Nonpharmaceutical interventions need to continue and be enforced to ensure high compliance so that the rate of immunity established by vaccination outpaces that induced by infections.
Keywords: COVID-19; United States; agent-based; agent-based model; capacity; cloud-based simulations; effective; impact; interaction; intervention; large-scale simulations; model; outcome; plan; scenario; simulation; strategy; vaccine; willingness.
©Junjiang Li, Philippe Giabbanelli. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 29.04.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
Impact of vaccine prioritization strategies on mitigating COVID-19: an agent-based simulation study using an urban region in the United States.BMC Med Res Methodol. 2021 Dec 5;21(1):272. doi: 10.1186/s12874-021-01458-9. BMC Med Res Methodol. 2021. PMID: 34865617 Free PMC article.
-
Impact of Vaccination and Nonpharmaceutical Interventions With Possible COVID-19 Viral Evolutions Using an Agent-Based Simulation.AJPM Focus. 2023 Oct 10;3(1):100155. doi: 10.1016/j.focus.2023.100155. eCollection 2024 Feb. AJPM Focus. 2023. PMID: 38130803 Free PMC article.
-
The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Sep 6;22(1):592. doi: 10.1186/s13063-021-05484-2. Trials. 2021. PMID: 34488843 Free PMC article.
-
Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types.J Manag Care Pharm. 2010 Apr;16(3):217-30. doi: 10.18553/jmcp.2010.16.3.217. J Manag Care Pharm. 2010. PMID: 20331326 Free PMC article. Review.
-
Impact of vaccination and non-pharmacological interventions on COVID-19: a review of simulation modeling studies in Asia.Front Public Health. 2023 Sep 25;11:1252719. doi: 10.3389/fpubh.2023.1252719. eCollection 2023. Front Public Health. 2023. PMID: 37818298 Free PMC article. Review.
Cited by
-
Activity-based epidemic propagation and contact network scaling in auto-dependent metropolitan areas.Sci Rep. 2021 Nov 22;11(1):22665. doi: 10.1038/s41598-021-01522-w. Sci Rep. 2021. PMID: 34811414 Free PMC article.
-
Predictive models for health outcomes due to SARS-CoV-2, including the effect of vaccination: a systematic review.Syst Rev. 2024 Jan 16;13(1):30. doi: 10.1186/s13643-023-02411-1. Syst Rev. 2024. PMID: 38229123 Free PMC article.
-
Assessing parameter sensitivity in a university campus COVID-19 model with vaccinations.Infect Dis Model. 2023 Jun;8(2):374-389. doi: 10.1016/j.idm.2023.04.002. Epub 2023 Apr 9. Infect Dis Model. 2023. PMID: 37064014 Free PMC article.
-
Evolution of Select Epidemiological Modeling and the Rise of Population Sentiment Analysis: A Literature Review and COVID-19 Sentiment Illustration.Int J Environ Res Public Health. 2022 Mar 9;19(6):3230. doi: 10.3390/ijerph19063230. Int J Environ Res Public Health. 2022. PMID: 35328916 Free PMC article. Review.
-
Agent-Based Simulation of the COVID-19 Epidemic in Russia.Her Russ Acad Sci. 2022;92(4):479-487. doi: 10.1134/S1019331622040219. Epub 2022 Sep 6. Her Russ Acad Sci. 2022. PMID: 36091848 Free PMC article.
References
-
- COVID Data Tracker. Centers for Disease Control and Prevention. [2021-01-21]. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days.
-
- Reese H, Iuliano AD, Patel NN, Garg S, Kim L, Silk BJ, Hall AJ, Fry A, Reed C. Estimated incidence of COVID-19 illness and hospitalization - United States, February-September, 2020. Clin Infect Dis. 2020 Nov 25;:ciaa1780. doi: 10.1093/cid/ciaa1780. http://europepmc.org/abstract/MED/33237993 - DOI - PMC - PubMed
-
- Rolfes MA, Foppa IM, Garg S, Flannery B, Brammer L, Singleton JA, Burns E, Jernigan D, Olsen SJ, Bresee J, Reed C. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018 Jan;12(1):132–137. doi: 10.1111/irv.12486. - DOI - PMC - PubMed
-
- Morlacco A, Motterle G, Zattoni F. The multifaceted long-term effects of the COVID-19 pandemic on urology. Nat Rev Urol. 2020 Jul;17(7):365–367. doi: 10.1038/s41585-020-0331-y. http://europepmc.org/abstract/MED/32377015 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

