Fidgetin-like 2 negatively regulates axonal growth and can be targeted to promote functional nerve regeneration
- PMID: 33872220
- PMCID: PMC8262307
- DOI: 10.1172/jci.insight.138484
Fidgetin-like 2 negatively regulates axonal growth and can be targeted to promote functional nerve regeneration
Abstract
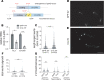
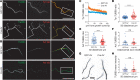
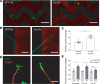
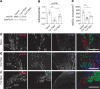
The microtubule (MT) cytoskeleton plays a critical role in axon growth and guidance. Here, we identify the MT-severing enzyme fidgetin-like 2 (FL2) as a negative regulator of axon regeneration and a therapeutic target for promoting nerve regeneration after injury. Genetic knockout of FL2 in cultured adult dorsal root ganglion neurons resulted in longer axons and attenuated growth cone retraction in response to inhibitory molecules. Given the axonal growth-promoting effects of FL2 depletion in vitro, we tested whether FL2 could be targeted to promote regeneration in a rodent model of cavernous nerve (CN) injury. The CNs are parasympathetic nerves that regulate blood flow to the penis, which are commonly damaged during radical prostatectomy (RP), resulting in erectile dysfunction (ED). Application of FL2-siRNA after CN injury significantly enhanced functional nerve recovery. Remarkably, following bilateral nerve transection, visible and functional nerve regeneration was observed in 7 out of 8 animals treated with FL2-siRNA, while no control-treated animals exhibited regeneration. These studies identify FL2 as a promising therapeutic target for enhancing regeneration after peripheral nerve injury and for mitigating neurogenic ED after RP - a condition for which, at present, only poor treatment options exist.
Keywords: Cytoskeleton; Neuroscience; Prostate cancer; Reproductive Biology; Urology.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

