The methodological quality of 176,620 randomized controlled trials published between 1966 and 2018 reveals a positive trend but also an urgent need for improvement
- PMID: 33872298
- PMCID: PMC8084332
- DOI: 10.1371/journal.pbio.3001162
The methodological quality of 176,620 randomized controlled trials published between 1966 and 2018 reveals a positive trend but also an urgent need for improvement
Abstract
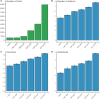
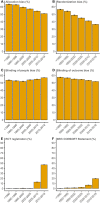
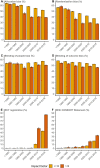
Many randomized controlled trials (RCTs) are biased and difficult to reproduce due to methodological flaws and poor reporting. There is increasing attention for responsible research practices and implementation of reporting guidelines, but whether these efforts have improved the methodological quality of RCTs (e.g., lower risk of bias) is unknown. We, therefore, mapped risk-of-bias trends over time in RCT publications in relation to journal and author characteristics. Meta-information of 176,620 RCTs published between 1966 and 2018 was extracted. The risk-of-bias probability (random sequence generation, allocation concealment, blinding of patients/personnel, and blinding of outcome assessment) was assessed using a risk-of-bias machine learning tool. This tool was simultaneously validated using 63,327 human risk-of-bias assessments obtained from 17,394 RCTs evaluated in the Cochrane Database of Systematic Reviews (CDSR). Moreover, RCT registration and CONSORT Statement reporting were assessed using automated searches. Publication characteristics included the number of authors, journal impact factor (JIF), and medical discipline. The annual number of published RCTs substantially increased over 4 decades, accompanied by increases in authors (5.2 to 7.8) and institutions (2.9 to 4.8). The risk of bias remained present in most RCTs but decreased over time for allocation concealment (63% to 51%), random sequence generation (57% to 36%), and blinding of outcome assessment (58% to 52%). Trial registration (37% to 47%) and the use of the CONSORT Statement (1% to 20%) also rapidly increased. In journals with a higher impact factor (>10), the risk of bias was consistently lower with higher levels of RCT registration and the use of the CONSORT Statement. Automated risk-of-bias predictions had accuracies above 70% for allocation concealment (70.7%), random sequence generation (72.1%), and blinding of patients/personnel (79.8%), but not for blinding of outcome assessment (62.7%). In conclusion, the likelihood of bias in RCTs has generally decreased over the last decades. This optimistic trend may be driven by increased knowledge augmented by mandatory trial registration and more stringent reporting guidelines and journal requirements. Nevertheless, relatively high probabilities of bias remain, particularly in journals with lower impact factors. This emphasizes that further improvement of RCT registration, conduct, and reporting is still urgently needed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Prayle AP, Hurley MN, Smyth AR. Compliance with mandatory reporting of clinical trial results on ClinicalTrials.gov: cross sectional study. BMJ. 2012;344:d7373. 10.1136/bmj.d7373 . - DOI - PubMed
-
- Bilandzic A, Fitzpatrick T, Rosella L, Henry D. Risk of Bias in Systematic Reviews of Non-Randomized Studies of Adverse Cardiovascular Effects of Thiazolidinediones and Cyclooxygenase-2 Inhibitors: Application of a New Cochrane Risk of Bias Tool. PLoS Med. 2016;13(4):e1001987. 10.1371/journal.pmed.1001987 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

