Diagnostic performance of anti-Zika virus IgM, IgAM and IgG ELISAs during co-circulation of Zika, dengue, and chikungunya viruses in Brazil and Venezuela
- PMID: 33872309
- PMCID: PMC8084345
- DOI: 10.1371/journal.pntd.0009336
Diagnostic performance of anti-Zika virus IgM, IgAM and IgG ELISAs during co-circulation of Zika, dengue, and chikungunya viruses in Brazil and Venezuela
Abstract
Background: Serological diagnosis of Zika virus (ZIKV) infection is challenging because of the antibody cross-reactivity among flaviviruses. At the same time, the role of Nucleic Acid Testing (NAT) is limited by the low proportion of symptomatic infections and the low average viral load. Here, we compared the diagnostic performance of commercially available IgM, IgAM, and IgG ELISAs in sequential samples during the ZIKV and chikungunya (CHIKV) epidemics and co-circulation of dengue virus (DENV) in Brazil and Venezuela.
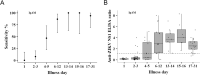
Methodology/principal findings: Acute (day of illness 1-5) and follow-up (day of illness ≥ 6) blood samples were collected from nine hundred and seven symptomatic patients enrolled in a prospective multicenter study between June 2012 and August 2016. Acute samples were tested by RT-PCR for ZIKV, DENV, and CHIKV. Acute and follow-up samples were tested for IgM, IgAM, and IgG antibodies to ZIKV using commercially available ELISAs. Among follow-up samples with a RT-PCR confirmed ZIKV infection, anti-ZIKV IgAM sensitivity was 93.5% (43/46), while IgM and IgG exhibited sensitivities of 30.3% (10/33) and 72% (18/25), respectively. An additional 24% (26/109) of ZIKV infections were detected via IgAM seroconversion in ZIKV/DENV/CHIKV RT-PCR negative patients. The specificity of anti-ZIKV IgM was estimated at 93% and that of IgAM at 85%.
Conclusions/significance: Our findings exemplify the challenges of the assessment of test performance for ZIKV serological tests in the real-world setting, during co-circulation of DENV, ZIKV, and CHIKV. However, we can also demonstrate that the IgAM immunoassay exhibits superior sensitivity to detect ZIKV RT-PCR confirmed infections compared to IgG and IgM immunoassays. The IgAM assay also proves to be promising for detection of anti-ZIKV seroconversions in sequential samples, both in ZIKV PCR-positive as well as PCR-negative patients, making this a candidate assay for serological monitoring of pregnant women in future ZIKV outbreaks.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: BW has received, outside the submitted work, fees for consulting and travel, in her role as a member of the Data Monitoring and Adjudication Committees for the Takeda dengue vaccine trials, and also personal fees from Roche for work on their Advisory Board on Severe Dengue.TJ has received, outside the submitted work, personal fees from Roche for work on their Advisory Board on Severe Dengue. All other authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

