Perovskites on Ice: An Additive-Free Approach to Increase the Shelf-Life of Triple-Cation Perovskite Precursor Solutions
- PMID: 33872471
- PMCID: PMC8251910
- DOI: 10.1002/cssc.202100332
Perovskites on Ice: An Additive-Free Approach to Increase the Shelf-Life of Triple-Cation Perovskite Precursor Solutions
Abstract
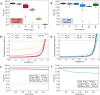
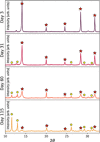
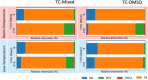
The development of stable perovskite precursor solutions is critical if solution-processable perovskite solar cells (PSCs) are to be practically manufacturable. Ideally, such precursors should combine high solution stability without using chemical additives that might compromise PSC performance. Here, it was shown that the shelf-life of high-performing perovskite precursors could be greatly improved by storing solutions at low-temperature without the need to alter chemical composition. Devices fabricated from solutions stored for 31 days at 4 °C achieved a champion power conversion efficiency (PCE) of 18.6 % (97 % of original PCE). The choice of precursor solvent also impacted solution shelf-life, with DMSO-based solutions having enhanced solution stability compared to those including DMF. The compositions of aged precursors were explored using NMR spectroscopy, and films made from these solutions were analysed using X-ray diffraction. It was concluded that the improvement in precursor solution stability is directly linked to the suppression of an addition-elimination reaction and the preservation of higher amounts of methylammonium within solution.
Keywords: perovskites; photovoltaics; solar cells; solution chemistry; solution processing.
© 2021 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
David Lidzey is a director and shareholder of the materials science company Ossila Ltd that retails equipment and materials (including perovskite inks) for photovoltaic device research.
Figures




References
-
- National Renewable Energy Laboratory, 2020, DOI: 10.1016/j.jet.2007.03.008.
-
- Eperon G. E., Stranks S. D., Menelaou C., Johnston M. B., Herz L. M., Snaith H. J., Energy Environ. Sci. 2014, 7, 982.
-
- McMeekin D. P., Sadoughi G., Rehman W., Eperon G. E., Saliba M., Horantner M. T., Haghighirad A., Sakai N., Korte L., Rech B., Johnston M. B., Herz L. M., Snaith H. J., Science 2016, 351, 151–155. - PubMed
-
- Zheng X., Wu C., Jha S. K., Li Z., Zhu K., Priya S., ACS Energy Lett. 2016, 1, 1014–1020.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

