Enantioselective Total Synthesis of (+)-EBC-23, a Potent Anticancer Agent from the Australian Rainforest
- PMID: 33872504
- PMCID: PMC8915666
- DOI: 10.1021/acs.joc.1c00172
Enantioselective Total Synthesis of (+)-EBC-23, a Potent Anticancer Agent from the Australian Rainforest
Abstract
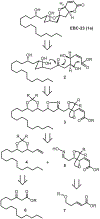
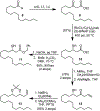
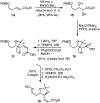
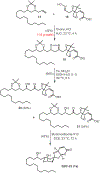
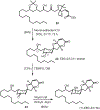
We describe here an enantioselective synthesis of (+)-EBC-23, a potent anticancer agent from the Australian rainforest. Our convergent synthesis features a [3+2] dipolar cycloaddition of an olefin-bearing 1,3-syn diol unit and an oxime segment containing 1,2-syn diol functionality as the key step. The segments were synthesized in a highly enantioselective manner using Noyori asymmetric hydrogenation of a β-keto ester and Sharpless asymmetric dihydroxylation of an α,β-unsaturated ester. Cycloaddition provided isoxazoline derivative which upon hydrogenolysis furnished the β-hydroxy ketone expediently. A one-pot, acid-catalyzed reaction removed the isopropylidene group, promoted spirocyclization, constructed the complex spiroketal lactone core, and furnished EBC-23 and its C11 epimer. The C11 epimer was also converted to EBC-23 by chemoselective oxidation and reduction sequence. The present synthesis provides convenient access to this family of natural products in an efficient manner.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
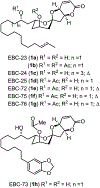
References
-
- Newman DJ; Cragg GM Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod 2020, 83, 770–803. - PubMed
-
- Newman DJ; Cragg GM Natural products as sources of new drugs over the last 25 years. J. Nat. Prod 2007, 70, 461–477. - PubMed
-
- Reddell PW; Gordon VA Spiroketals WIPO Patent WO2007070984A12007.
-
- Reddell PW; Gordon VA Spiroketals U.S. Patent US8013012B22011.
-
- Dong L; Gordon VA; Grange RL; Johns J; Parsons PG; Porzelle A; Reddell P; Schill H; Williams CM Structure and Absolute Stereochemistry of the Anticancer Agent EBC-23 from the Australian Rainforest. J. Am. Chem. Soc 2008, 130, 15262–15263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

