Discovery of a AHR pelargonidin agonist that counter-regulates Ace2 expression and attenuates ACE2-SARS-CoV-2 interaction
- PMID: 33872570
- PMCID: PMC8052506
- DOI: 10.1016/j.bcp.2021.114564
Discovery of a AHR pelargonidin agonist that counter-regulates Ace2 expression and attenuates ACE2-SARS-CoV-2 interaction
Abstract
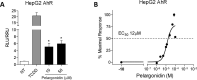
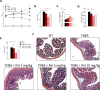
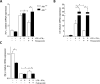
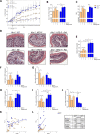
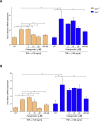
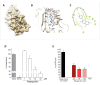
The severe acute respiratory syndrome (SARS)-CoV-2 is the pathogenetic agent of Corona Virus Induced Disease (COVID)19. The virus enters the human cells after binding to the angiotensin converting enzyme (ACE)2 receptor in target tissues. ACE2 expression is induced in response to inflammation. The colon expression of ACE2 is upregulated in patients with inflammatory bowel disease (IBD), highlighting a potential risk of intestinal inflammation in promoting viral entry in the human body. Because mechanisms that regulate ACE2 expression in the intestine are poorly understood and there is a need of anti-SARS-CoV-2 therapies, we have settled to investigate whether natural flavonoids might regulate the expression of Ace2 in intestinal models of inflammation. The results of these studies demonstrated that pelargonidin activates the Aryl hydrocarbon Receptor (AHR) in vitro and reverses intestinal inflammation caused by chronic exposure to high fat diet or to the intestinal braking-barrier agent TNBS in a AhR-dependent manner. In these two models, development of colon inflammation associated with upregulation of Ace2 mRNA expression. Colon levels of Ace2 mRNA were directly correlated with Tnf-α mRNA levels. Molecular docking studies suggested that pelargonidin binds a fatty acid binding pocket on the receptor binding domain of SARS-CoV-2 Spike protein. In vitro studies demonstrated that pelargonidin significantly reduces the binding of SARS-CoV-2 Spike protein to ACE2 and reduces the SARS-CoV-2 replication in a concentration-dependent manner. In summary, we have provided evidence that a natural flavonoid might hold potential in reducing intestinal inflammation and ACE2 induction in the inflamed colon in a AhR-dependent manner.
Keywords: ACE2; Ahr; Intestinal inflammation; NF-kB; Pelargonidin; SARS-CoV-2; TNF-α.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

