Comparison of four PCR and two point of care assays used in the laboratory detection of SARS-CoV-2
- PMID: 33872650
- PMCID: PMC8051013
- DOI: 10.1016/j.jviromet.2021.114165
Comparison of four PCR and two point of care assays used in the laboratory detection of SARS-CoV-2
Abstract
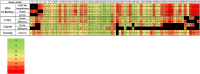
Seeing the global emergence and the lack of a definitive cure for COVID-19, it is essential to find the most sensitive and specific detection method to identify infected patients in a timely manner. Our paper aims to compare the clinical sensitivity of different commercial RT-qPCR (Genesig, 1copy, DNA-Techonolgy and Charité primer-probe sets), isothermal PCR (Ustar Isothermal Amplification-Real Time Fluorescent Assay) and immunochromatographic antigen detection (BIOCREDIT COVID-19 Ag) assays developed to use in laboratory diagnosis of COVID-19. A total of 119 nasopharyngeal swab specimens were collected from symptomatic patients. A subset of samples, positive with two RT-qPCR assays were then tested with isothermal PCR and rapid antigen tests. Of the 119 specimens, 65 were positive by at least two PCR assays. All PCR assays showed substantial or perfect match, although some variations in the clinical performance was observed. Of the 37 and 32 remnant nasopharyngeal samples positive by RT-qPCR, respectively, three were positive by the BIOCREDIT COVID-19 Ag and 14 were detected by the isothermal amplification assay. In conclusion, in the clinical settings we recorded that each of the RT-qPCR assays was superior to other test formats, in particular, the routine use of the DNA-technology assay is recommended. Although alternative recommendations exist, we belive that the use of isothermal amplifiaction assays and antigen rapid tests for COVID-19 diagnosis can only serve as adjuncts while awaiting the PCR result because of their high false-negative rate.
Keywords: COVID-19; Laboratory diagnostics; RT-qPCR; SARS CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Artesi M., Bontems S., Gobbels P., Franckh M., Maes P., Boreux R., Meex C., Melin P., Hayette M.P., Bours V., Durkin K. A recurrent mutation at position 26340 of SARS-CoV-2 is associated with failure of the e gene quantitative reverse Transcription-PCR utilized in a commercial dual-target diagnostic assay. J. Clin. Microbiol. 2020:58. - PMC - PubMed
-
- Cascella M R.M., Cuomo A., et al. Features, evaluation, and treatment of coronavirus (COVID-19) StatPearls. 2021 - PubMed
-
- Christian Drosten V.C., Bleicker Tobias, Brünink Sebastian. 2020. Diagnostic Detection of 2019-nCoV by Real-time RT-PCR.https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?s... Accessed at. - PMC - PubMed
-
- ECDC . 2020. COVID-19 Situation Update Worldwide, As of 30 March 2021. Accessed at: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases 2020.
-
- FDA . 2020. Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices.https://www.fda.gov/medical-devices/emergency-use-authorizations-medical... Accessed at.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

