Full length RAG2 expression enhances the DNA damage response in pre-B cells
- PMID: 33873062
- PMCID: PMC8169632
- DOI: 10.1016/j.imbio.2021.152089
Full length RAG2 expression enhances the DNA damage response in pre-B cells
Abstract
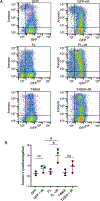
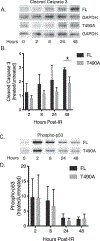
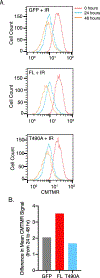
V(D)J recombination by the RAG1 and RAG2 protein complex in developing lymphocytes includes DNA double strand break (DSB) intermediates. RAG2 undergoes export from the nucleus and enrichment at the centrosome minutes following production of DSBs by genotoxic stress, suggesting that RAG2 participates in cellular responses to DSBs such as those generated during V(D)J recombination. To determine the effect of RAG2 expression on cell viability following DSB generation, we measured pre-B cells that expressed either full length (FL) wild-type RAG2, or a T490A mutant of RAG2 that has increased stability and fails to undergo nuclear export following generation of DSBs. Each RAG2 construct was labeled with GFP at the N-terminus. Compared to the T490A mutant, cells expressing FL RAG2 exhibited elevated apoptosis by 24 h following irradiation, and this coincided with a greater amount of Caspase 3 cleavage measured in cell lysates. Pre-B cells expressing either RAG2 protein exhibited similar increases in phospho-p53 levels following irradiation. Interestingly, FL RAG2-expressing cells exhibited elevated division relative to the T490A clone beginning ~24 h following irradiation, as well as an increased percentage of cells proceeding through mitosis, suggesting an improved rate of recovery following the initial burst in apoptosis. Altogether, these data show that FL RAG2, but not its stable nuclear export-defective T490A mutant, participates in pre-B cell decisions between apoptosis versus DNA repair and cell cycle progression following DNA damage.
Keywords: Apoptosis; Cell cycle; DNA damage response; DNA repair; Genotoxic stress; Pre-B cells; RAG1; RAG2; V(D)J recombination.
Copyright © 2021 Elsevier GmbH. All rights reserved.
Figures

References
-
- Gellert M (2002) V(D)J recombination: RAG proteins, repair factors, and regulation. Annu. Rev. Biochem. 71, 101–132 - PubMed
-
- Schatz DG, and Ji Y (2011) Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 11, 251–263 - PubMed
-
- Schatz DG, and Swanson PC (2011) V(D)J recombination: mechanisms of initiation. Annu Rev Genet 45, 167–202 - PubMed
-
- Rooney S, Chaudhuri J, and Alt FW (2004) The role of the non-homologous end-joining pathway in lymphocyte development. Immunol Rev 200, 115–131 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

